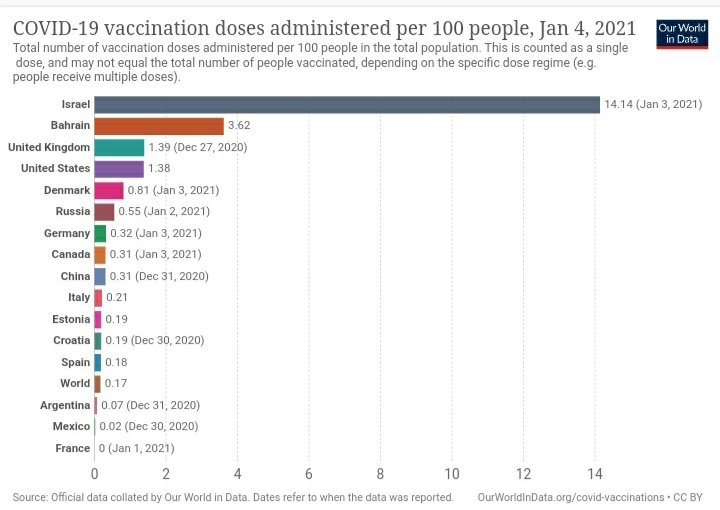
In an earlier analysis, we had seen how the TPR and CPM of Kerala, spiked post September, 2020.
Results of the 3 national sero surveys give clues to interpret this.
➡️ As of August 2020, KL had a seroprevalence of only 0.8% compared to national avg. of 6.6%.
1/5
Results of the 3 national sero surveys give clues to interpret this.
➡️ As of August 2020, KL had a seroprevalence of only 0.8% compared to national avg. of 6.6%.
1/5
https://twitter.com/covid19indiaorg/status/1359133066678079488

➡️ This relatively low prevalence in KL correlates with the lower case load seen in the state till August.
➡️ In Dec, the prevalence saw a multi fold increase to 11.6% (national 21%) from 0.8% in August. This is reflected in the significant spike seen in KL from September.
2/5
➡️ In Dec, the prevalence saw a multi fold increase to 11.6% (national 21%) from 0.8% in August. This is reflected in the significant spike seen in KL from September.
2/5

➡️ It is notable that in Aug, KL had a relatively low prevalence of 0.8% - reflecting the initial control of spread.
➡️ It left a high % of population vulnerable to infection, which would have contributed to the spike post August. Furthered by local body elections and Onam.
3/5
➡️ It left a high % of population vulnerable to infection, which would have contributed to the spike post August. Furthered by local body elections and Onam.
3/5
➡️ How many of the actual cases did KL identify and report?
As per the 3rd serosurvey, it's estimated that KL reported 1 in 6 actual cases. Nationally, only 1 in 30 cases have been reported.
➡️ With only 11.6% prevalence as of December,
4/5
As per the 3rd serosurvey, it's estimated that KL reported 1 in 6 actual cases. Nationally, only 1 in 30 cases have been reported.
➡️ With only 11.6% prevalence as of December,
4/5
KL still runs the risk of a high % of uninfected population. With the state elections coming up in next few months, this risk will rear it's head again.
📣 ICMR serosurveys were done in 3 districts of KL. The state will be carrying out serosurvey across all districts.
5/5
📣 ICMR serosurveys were done in 3 districts of KL. The state will be carrying out serosurvey across all districts.
5/5
• • •
Missing some Tweet in this thread? You can try to
force a refresh