Is the health ministry doing enough to inspect the cause of COVID-19 surge?
To change strategy, we need to identify & sequence
Dr Vardhan claims SARS-CoV-2 genomics consortium is effectively processing the samples of the double variant.
Lets fact check:
#COVID19India #Thread
To change strategy, we need to identify & sequence
Dr Vardhan claims SARS-CoV-2 genomics consortium is effectively processing the samples of the double variant.
Lets fact check:
#COVID19India #Thread
https://twitter.com/drharshvardhan/status/1383082011699519489
1. Yesterday, Indian State health officials said the double variant’s role cannot be ruled out in Maharashtra’s second wave. The state is recording over 50,000 new cases every day and has 5.64 lakh active cases, half of India’s Covid burden.
indianexpress.com/article/cities…
indianexpress.com/article/cities…
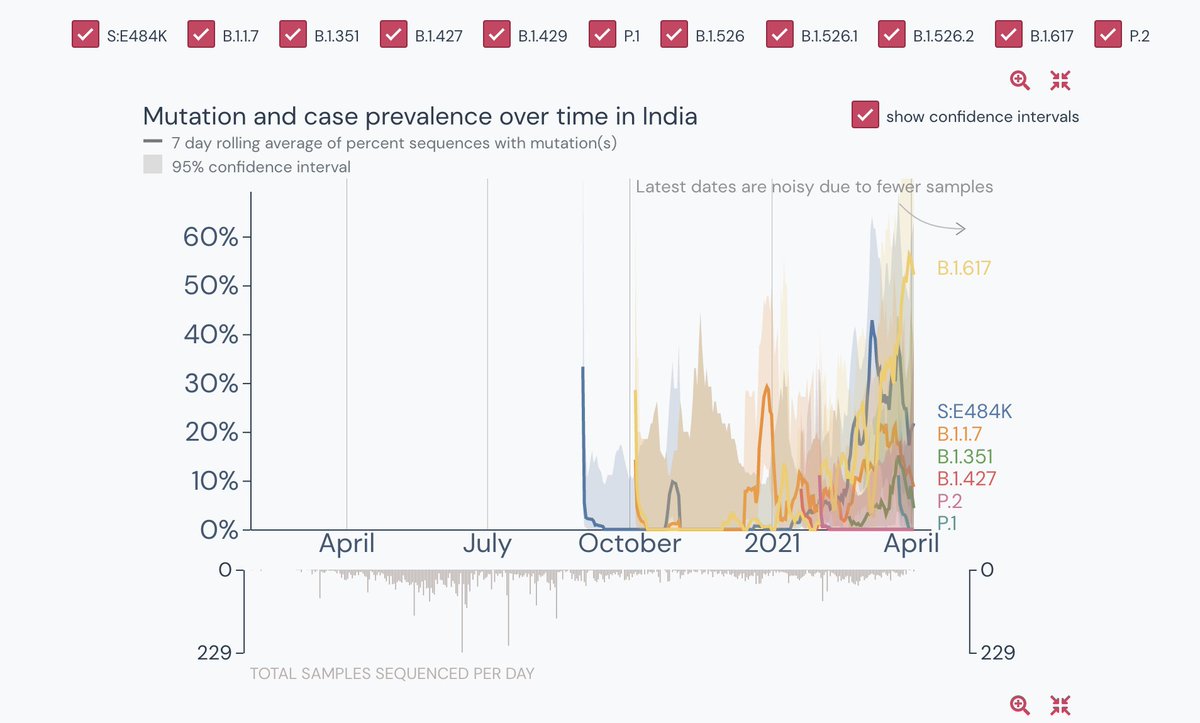
2. The B.1.617 variant has two mutations, E484Q & L452R. Both separately found in many other variants, but together, it was found for the first time in India on 5 Oct 2020
Both mutations are found in the spike protein that helps the virus to bind to our cell’s receptors....
Both mutations are found in the spike protein that helps the virus to bind to our cell’s receptors....
3. Together, the double mutation of B.1.617 is more infectious (spreads faster) & is deadlier as it evades antibodies.
However, is it really that this double whopper has been analysed with 13K samples? Lets access:
The sampling efficiency is defined by
1. Number
2. Rate
However, is it really that this double whopper has been analysed with 13K samples? Lets access:
The sampling efficiency is defined by
1. Number
2. Rate
4. How efficient was India data reporting since 10th Jan 2020?
4.1. The number of genome sequencing samples:
Genomes = 7,778
Reported cases = 14,291,917
4.2. Rate of reporting:
% of cases sequenced & shared = 0.054 %
Median days to data deposition = 64 days (>2 months)
4.1. The number of genome sequencing samples:
Genomes = 7,778
Reported cases = 14,291,917
4.2. Rate of reporting:
% of cases sequenced & shared = 0.054 %
Median days to data deposition = 64 days (>2 months)
Source of Tweet 4:
GISAID website Last updated 16 April 2021 20:11hrs UTC
GISAID website Last updated 16 April 2021 20:11hrs UTC
5. Infact despite this high surge, this sampling report is based only on 265 total samples, last submitted on the 3rd of April 2020.
Source: Outbreak.info
Source: Outbreak.info

6. While most of the states, apart from Maharashtra, Karnataka, Andhra, Telangana & WB have not even sequenced more than 5 samples of the new variant in the entire state. The greyed states mean they have sampled less than 5. 

7. When all the variants are combined, the sampling in the entire country was only 155 in the last 30 days- a consistent low from feb 20. See the surge in the double variant. Also a small portion 4% is unknown but its a lot given the sample size.
Data from the 60 & 30 days.

Data from the 60 & 30 days.
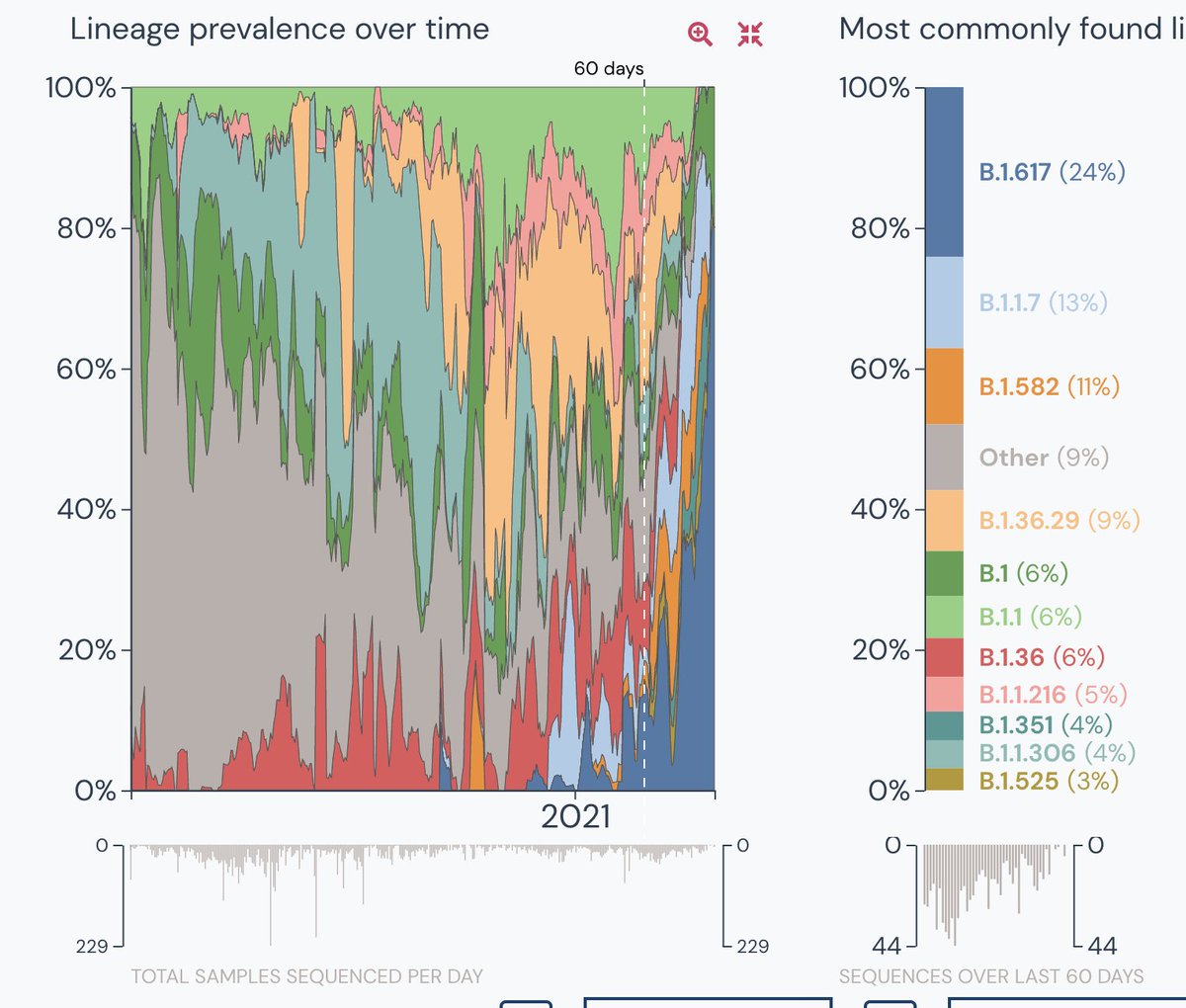
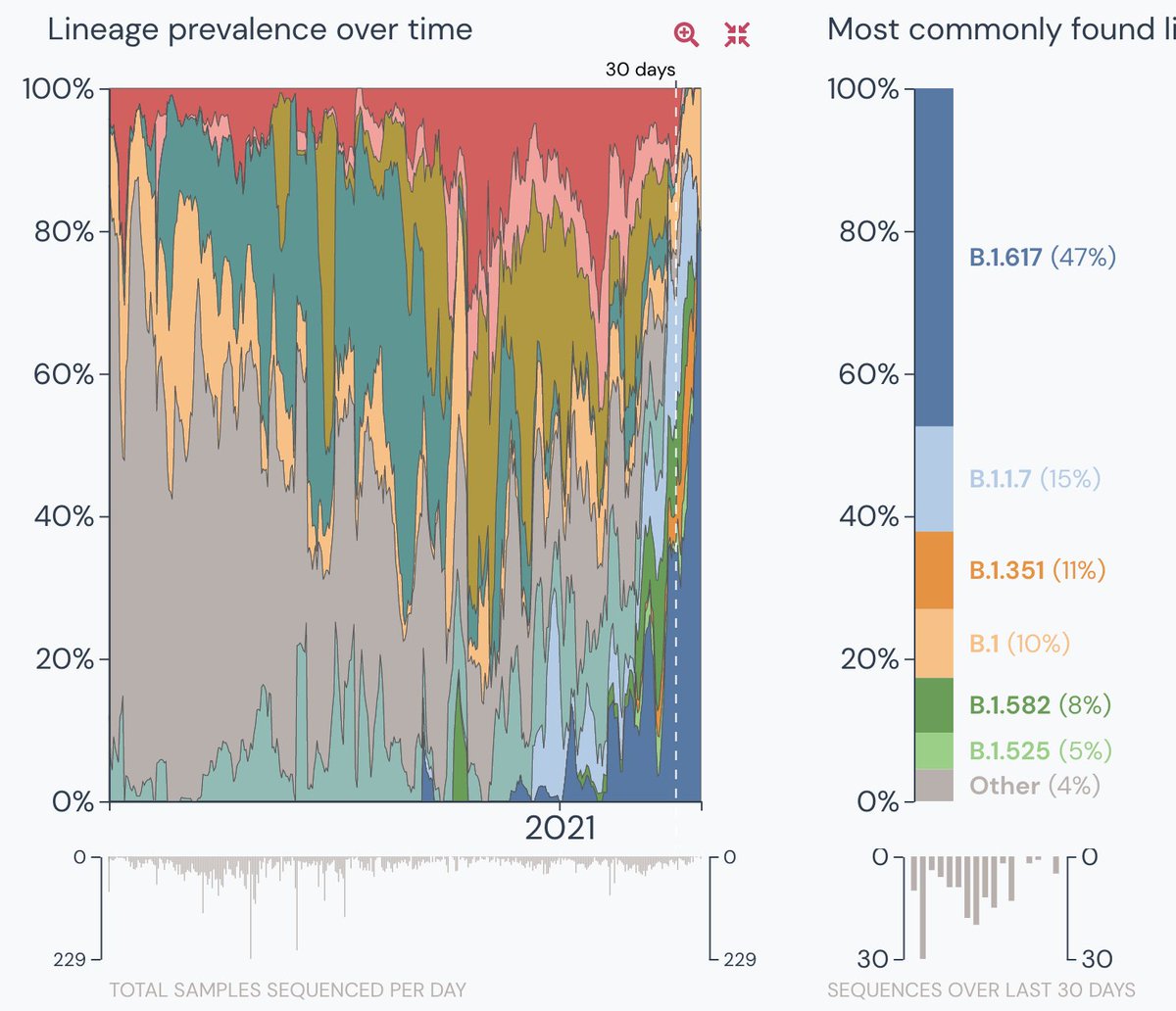
8. In comparison, respective data from
Country: UK & USA
Genomes shared: 368,740 & 292,415
Reported cases: 4,380,980 & 31,103,006
% of cases sequenced+shared: 8.42 & 0.94
Median days to deposition: 17 & 30
Very very large proportion. See the charts below

Country: UK & USA
Genomes shared: 368,740 & 292,415
Reported cases: 4,380,980 & 31,103,006
% of cases sequenced+shared: 8.42 & 0.94
Median days to deposition: 17 & 30
Very very large proportion. See the charts below
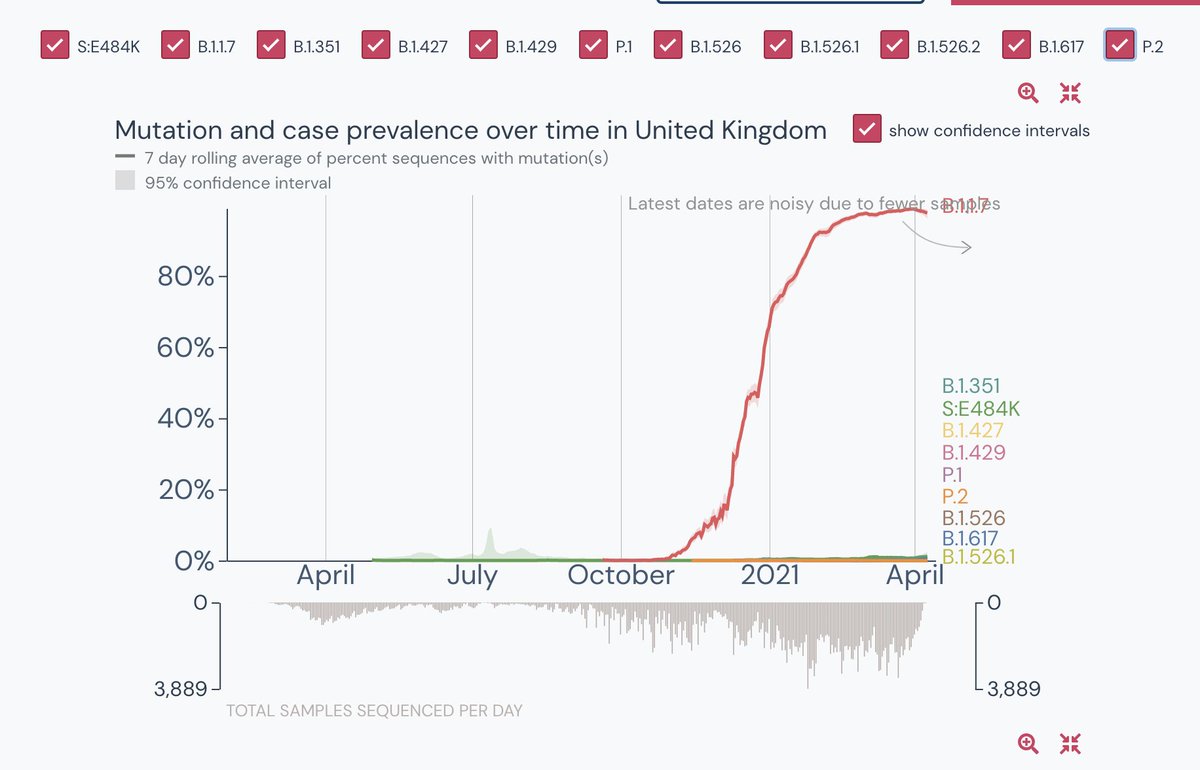
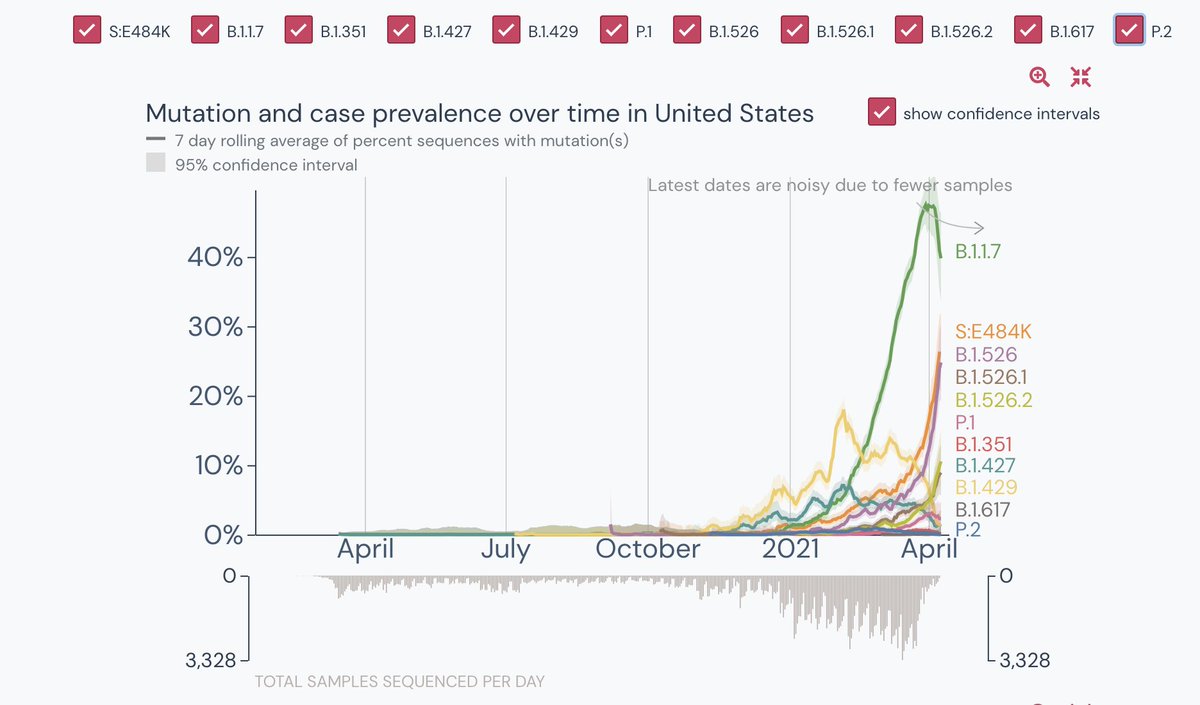
For a country leading with COVID-19, a dataset of 7K + genome sequencing is not even close to average. Again, to cure, we need to identify. And to identify, we need to increase genome surveillance.
Info GISAID.org , Covariants.org & Outbreak.info
Info GISAID.org , Covariants.org & Outbreak.info
• • •
Missing some Tweet in this thread? You can try to
force a refresh