JUST OUT in @NEJM: Safety / Efficacy data for @novavax COVID-19 vaccine.
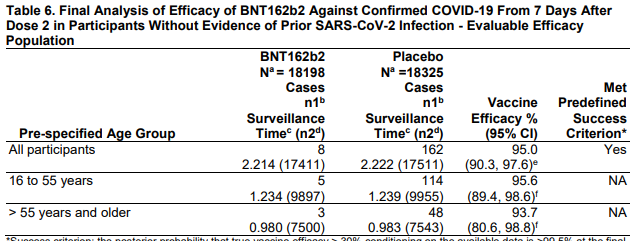
-89.7% efficacy against symptomatic COVID-19
-86.3% against alpha variant (B.1.1.7)
-96.4% against other variants
-NO hospitalizations, NO deaths in vaccine arm
DOI: 10.1056/NEJMoa2107659
Brief thread:
-89.7% efficacy against symptomatic COVID-19
-86.3% against alpha variant (B.1.1.7)
-96.4% against other variants
-NO hospitalizations, NO deaths in vaccine arm
DOI: 10.1056/NEJMoa2107659
Brief thread:

@NEJM @Novavax The @novavax vaccine is a (spike) protein-based vaccine, with an adjuvant that psychs up the immune system. This is older tech, like pertussis / Hep B vaccines - and may (?!) be more acceptable to those who are a bit nervous about mRNA vaccines. 
@NEJM @Novavax This report is on the UK trial - 15,000 participants. Half got placebo, half the vaccine (which is 2 doses, 21 days apart).
UK means this is mostly white people, though US data seems similar.
UK means this is mostly white people, though US data seems similar.

@NEJM @Novavax Efficacy 7 days after dose two is solid at near 90%. Starting the clock after dose 1 is, as expected, not as good - 70% or so. We'll need both doses I think. 

@NEJM @Novavax Trial was conducted at B.1.17 (Alpha) variant was kicking off in the UK. Efficacy very good here as well. Great news.
Don't get too excited about the high efficacy against "other variants" though - no Delta in the population at the time this trial was going.

Don't get too excited about the high efficacy against "other variants" though - no Delta in the population at the time this trial was going.


@NEJM @Novavax On to safety...
Systemic adverse events worse in vaccine group, particularly after dose 2 which we've come to expect. Most not severe.
Systemic adverse events worse in vaccine group, particularly after dose 2 which we've come to expect. Most not severe.

@NEJM @Novavax A bit of a different side-effect profile than the @pfizer and @moderna shots. Less fever here. But headache, fatigue, muscle pain pretty common. Feels like some folks might need to take a day off of work after this. 

@NEJM @Novavax @pfizer @Moderna Digging into the supplement for details on the serious events. 44 in vax group, 44 in placebo group. Nothing standing out as a "signal" on my first pass. Here's the cardiac data for those worried about this: 

@NEJM @Novavax @pfizer @Moderna People always ask about pregnancy. Pregnant women excluded (as ever) from this trial. Some women became pregnant, but I can't find a hard count. One miscarriage reported in the vax group.
@NEJM @Novavax @pfizer @Moderna Take home?
-Solid SOLID efficacy for this protein-based vaccine.
-No data on efficacy against delta though
-Side effect profile seems on par with mRNA vaccines
-No major safety signals.
-Solid SOLID efficacy for this protein-based vaccine.
-No data on efficacy against delta though
-Side effect profile seems on par with mRNA vaccines
-No major safety signals.
@NEJM @Novavax @pfizer @Moderna I assume this will get an EUA. The big question is whether this more familiar vaccine tech will move the needle and get some folks into the vaccinated camp. I hope so.
/Thread.
/Thread.
• • •
Missing some Tweet in this thread? You can try to
force a refresh