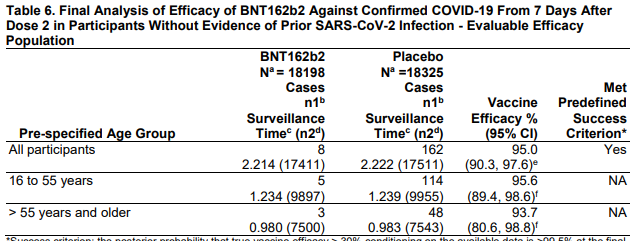
Efficacy overall (that's the 95% you keep hearing about) and stratified by age group.
Looks similar whether > or < 55 years of age. 3/
Looks similar whether > or < 55 years of age. 3/
More subgroups. Looks efficacious across multiple slices of the data (though smaller numbers make the precision of these estimates low). 4/ 

DAMN. Red line is cumulative cases in placebo. Blue is cumulative in vaccine group. Separation at 14 days which, interestingly, is BEFORE dose 2. But don't get carried away, the overall benefit occurs here after dose 2. Still - might be partial protection with 1 dose. 5/ 

What about prevention of SEVERE COVID-19. Only 3 cases in placebo group, 1 in vaccine group - so overall efficacy 66% with a gigantic confidence interval. Don't be disheartened - this is lack of data not lack of efficacy.
6/
6/

This looks at efficacy from the point of dose 1, not 7 days after dose 2, so this as expected doesn't look as good. You can get infected a day after the first shot - not enough time to build immunity. Critical to continue to be careful until AFTER dose 2.
7/
7/

(Actually you should stay careful no matter what since this is not a 100% effective vaccine - no vaccine is).8/
Adverse events.
Lots to parse here.
126 serious events in vax group, 101 in placebo. Not bad.
But LOTS of injection site reactions - this shoudl be expect4ed. Systemic AEs (fever, etc) also seem common.
Overall good news. 9/
Lots to parse here.
126 serious events in vax group, 101 in placebo. Not bad.
But LOTS of injection site reactions - this shoudl be expect4ed. Systemic AEs (fever, etc) also seem common.
Overall good news. 9/

More granular AE data - these are unsolicited - ie stuff the participants brought up on their own.
Take home is to expect injection site reactions, fatigue, fever, chills, muscle pain - but most patients not bad enough to bother telling study coordinators about it. 10/
Take home is to expect injection site reactions, fatigue, fever, chills, muscle pain - but most patients not bad enough to bother telling study coordinators about it. 10/

6 deaths (4 placebo, 2 vax).
Vax group was one heart attack long after vax and one death from "arteriosclerosis" 3 days after dose 1. Not sure what that latter one means? Would like a bit more detail here.
11/
Vax group was one heart attack long after vax and one death from "arteriosclerosis" 3 days after dose 1. Not sure what that latter one means? Would like a bit more detail here.
11/

In terms of serious adverse events - rates are nice and low.
Eyebrows raised at a higher number of appendicitis cases in vax vs. placebo group, but this could just be a statistical fluke. I guess something to watch for during roll-out.
12/
Eyebrows raised at a higher number of appendicitis cases in vax vs. placebo group, but this could just be a statistical fluke. I guess something to watch for during roll-out.
12/

All in all, this is REALLY encouraging data. I'll be very surprised if the EUA isn't granted on 12/10. And given this - I would 100% get this vaccine myself.
13/
13/
Link to the document this tweet thread is based on fda.gov/media/144245/d…
Thanks everyone. Stay safe! 14/14.
Thanks everyone. Stay safe! 14/14.
A bit more detail in video format (my first live stream cause this is exciting!).
• • •
Missing some Tweet in this thread? You can try to
force a refresh