The highly anticipated study of J&J booster antibody responses is out. (Note this is not the 2-dose Ph3 clinical trial.) As mentioned in press release a 2nd J&J shot 6mo after the first bumps antibody levels ~9x.
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…

Also side effects seem milder than after the first dose. These are great results and hopefully J&J boosters can be approved soon.
So how does JJ+JJ compares= to RNA+RNA? Recall antibodies after JJ had been found to be 7x lower than after RNA+RNA, below (30 vs ~200 units)
biorxiv.org/content/10.110…
biorxiv.org/content/10.110…

Recall as well even earlier studies had, by normalizing antibody levels to those found in convalescent patient controls, estimated the RNA+RNA vaccines to elicit 6-8x higher antibodies then JJ, and antibody levels correlate with efficacy against disease
nature.com/articles/s4159…
nature.com/articles/s4159…
Thus the 7-9x boost we get from a 2nd J&J shot likely brings its efficacy vs disease to the peak of the RNA response, which is about 88% protection against Delta. This compares to an unknown efficacy of 1-shot J&J vs Delta, but possibly ~67% if it is between Beta and original.
An important finding is that at 169 days, some vaccinees over 65yo (14-36% in 3 groups studied) had detectable responses at all. So the titers described are only among responders; to get means across all participants you have to multiply by 0.64-0.86 (based on how they worded it)
But I'm not sure about the numbers if you look carefully. In one group, there were n=25 ≥65yo, but the figure says 86% had detectable responses. Should be 84% if 21/25, or 88% if 22/25. So what is 86%? Makes you want to double-check numbers. 
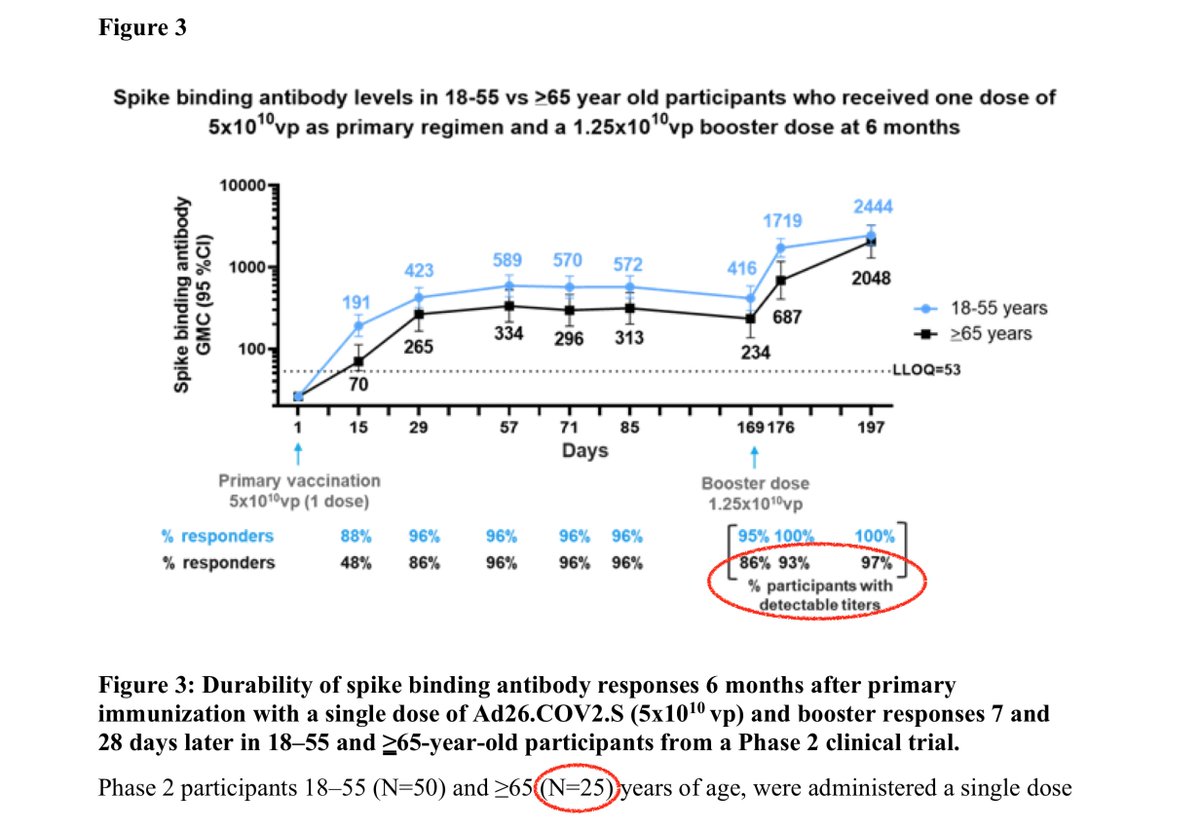
In any case, it seems most of the non-responders in this group did successfully create antibodies after the boost, as 86% response rate increased to 97% response. But again it should be 96% if it's 24/25.
So overall among #JnJers, who needs a booster? I'd suggest if you're >65yo or immunocompromised or you have elementary school kids and don't want them causing an outbreak at school, then the 67 vs 87% protection would be worth it.
If we're trying to suppress disease as much as possible, to secondarily protect young kids who can't get vaccines or older/immunocompromised who don't respond well to boosters, then boosts for everyone may be useful. But one could argue that the same doses are better used abroad
BTW as others have pointed out this study, while showing promising results, is not exactly a paragon of quality. Several clear shortcomings:
1. they look at anti-spike antibodies by ELISA, but don't perform virus neutralization assays or any T cell assays. This is odd; if you have the blood you should perform as many assays as you can from it. Especially when T cells is supposed to be the strength of J&J's vaccine.
On this point, note we did see a set of antibody and T cell results from their Phase 2 two-dose trial, where there was a 2.6- to 2.9-fold jump in antibodies after the second dose, 1 month after the first.
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
2. n of 17 for boosters just isn't that impressive, when there are 14 million Americans they could recruit from. You could probably get 17 volunteers just by canvassing your local supermarket.
Expanding n and the types of assays should not normally be an issue organizationally or financially for J&J. If they wanted to only use people from their trials, there were 1000s enrolled since 2020.
Suggests maybe this study was performed in a rush in response to recent questions about what options J&J recipients had?
3. They didn't show individual trajectories, but other studies have been doing that. Should be an easy fix. One example below
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
4. They look at 18-55yo and ≥65yo. What happened to 55-64yo? Just a bit strange.
In post#7 above, meant to write "some vaccinees over 65yo (14-36% in 3 groups studied) had *NO* detectable responses at all. So the titers described are only among responders; to get means across all participants you have to multiply by 0.64-0.86 (based on how they worded it)"
• • •
Missing some Tweet in this thread? You can try to
force a refresh