🧵We discussed how mucosal antibodies function to block SARSCoV2 infection. A study on this just posted from my Stanford colleague Michal Tal and UToronto collaborator Jennifer Gommerman.
Interesting: RNA and J&J make Abs in saliva, but neutralizing levels only detected with RNA
Interesting: RNA and J&J make Abs in saliva, but neutralizing levels only detected with RNA
2) First, as a reminder, the role of mucosal antibodies (thought mostly to be IgA, but we'll revisit that in a moment) is to block viruses, once they land on your mucous membranes, from entering your cells.
https://twitter.com/michaelzlin/status/1432056596348108801
4) First they looked at plasma levels of neutralizing antibodies in J&J vs convalescent (previously infected) plasma. The assay uses VSV (a harmless virus we also work with) expressing the SARSCoV2 spike protein to mediate cell attachment and entry into cells in culture.
5) You mix some human plasma into it and see if it blocks entry. If it blocks, it means there are neutralizing antibodies in the plasma. You know it's specific to vaccination because you have control plasma that doesn't block.
6) You then dilute the plasma further to see what dilution stops blocking. If there are high levels of antibodies, you can dilute a lot and still block. If there are low levels of antibodies, you lose blocking with a lower dilution. Hope that makes sense.
7) What do we expect? Well you may recall this paper I keep citing. It says plasma Ab levels in J&J (Ad26CoV2S) trial participants were about 0.5x of convalescent plasma. So we expect J&J neutralization to end at ~half the dilution of convalescent plasma
nature.com/articles/s4159…
nature.com/articles/s4159…
8) Indeed this is what the Tal lab observed (I moved panels around). Plasma was >50% neutralizing up to 1:400 in J&J vs 1:800 convalescent. Always nice to see the same thing in the real world as seen in clinical trials.
Notice the variability. These assays are not easy to do.
Notice the variability. These assays are not easy to do.

9) Having established the neutralization assay works, they then looked at neutralization by saliva. Note there's some mild neutralization activity in saliva without vaccination. But 1 and 2 months after J&J vaccine, there's no detectable additional neutralizing activity in saliva 

10) In contrast, in previously infected (convalescent) people, there was detectable neutralizing activity in saliva beyond control levels. 

11) Also, after vaccination by a RNA vaccine (Pfizer and Moderna samples grouped together for analysis, but split into 1-dose, 2-dose with low plasma abs, or 2-dose with high plasma abs) there is detectable neutralizing activity in saliva beyond non-vax controls. 

12) A caveat is that saliva from RNA-vaccinated were collected using a different kit than from convalescent and J&J, so RNA neutralizing titers may not be compared directly with convalescent or J&J titers. But each can be compared to their similarly collected controls.
13) So are the saliva antibodies seen in RNA IgA? Surprisingly there was both IgG and IgA. IgG isn't supposed to be there, but the authors speculate that high levels of IgG in the blood and interstitial tissues can leak out into mucous or saliva. 
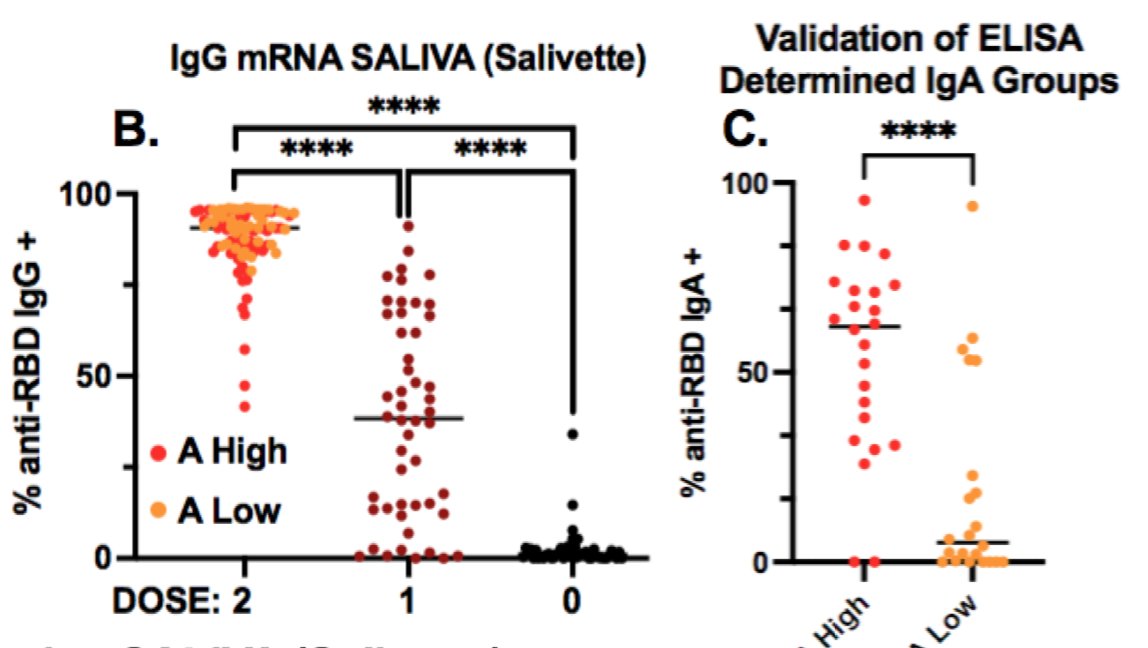
14) They were also able to detect a little bit of IgG in J&J saliva, but not IgA (asterisks mean statistically significant difference, ns means non-significant) 

15) So J&J elicits neutralizing antibodies in saliva lower than previous infection (undetectable neutralization in this study). They may be there though, as IgG can be detected.
RNA vax elicit neutralizing Abs in saliva, and these are both IgG and IgA.
Nice work by @ImmunoFever
RNA vax elicit neutralizing Abs in saliva, and these are both IgG and IgA.
Nice work by @ImmunoFever
• • •
Missing some Tweet in this thread? You can try to
force a refresh