The South African trial has finally revealed something about J&J effectiveness against Delta disease, and it is "about half".
No precise #, just a response to an interview question
Low protection and lack of clarity by trial organizers not surprising
bloomberg.com/news/articles/…
No precise #, just a response to an interview question
Low protection and lack of clarity by trial organizers not surprising
bloomberg.com/news/articles/…
You may recall on August 6 the SA trial announced 71% effectiveness against Delta hospitalization, but didn't say anything about effectiveness against disease, which is the default metric and the one used if you are gonig to report only one number.
Some surmised that effectiveness against Delta disease must be very unimpressive, or else it would have been reported alongside the hospitalization numbers on 8/6 (which were already unimpressive).
This has been proven correct.
This has been proven correct.
That the SA trial is only willing to issue positive statements is understandable because they received the J&J vaccine free to provide to their HCWs. Not good to bite the hand that feeds, and better to avoid complaints re the choice of vaccine.
All understandable, but we (and the press) just need to factor this into the interpretation of their statements.
Instead the press has been dutifully repeating the one-sided statements from the SA trial, e.g. below
wsj.com/articles/j-j-v…
Instead the press has been dutifully repeating the one-sided statements from the SA trial, e.g. below
wsj.com/articles/j-j-v…
I and others pointed out that 71% protection for hospitalization by J&J is far lower than the >90% for Pfizer and Moderna vaccines
https://twitter.com/profshanecrotty/status/1423684812309626889
Indeed anything less than 90% is being considered unacceptable to RNA recipients. Indeed FDA head Janet Woodcock recently stated the goal of boosters for Pfizer and Moderna vaccinees is to prevent any drop in effectiveness against hospitalization.
webmd.com/coronavirus-in…
webmd.com/coronavirus-in…
Yet J&J produces only 71% protection against hospitalization (vs >90% for RNA vaccines) and ~50% protection against disease (vs ~80% for RNA vaccines at this point in time in the US). Quite clearly it is J&J vaccinees who need the boost first, not RNA vaccinees.
@PaulSaxMD and I supported J&J recipients getting boosters at the same schedule as RNA vaccines. We pointed out J&J efficacy vs Delta was unknown at the time of writing, but the South Africa hospitalizations and breakthrough indicators suggested it's worse
nytimes.com/2021/08/29/opi…
nytimes.com/2021/08/29/opi…
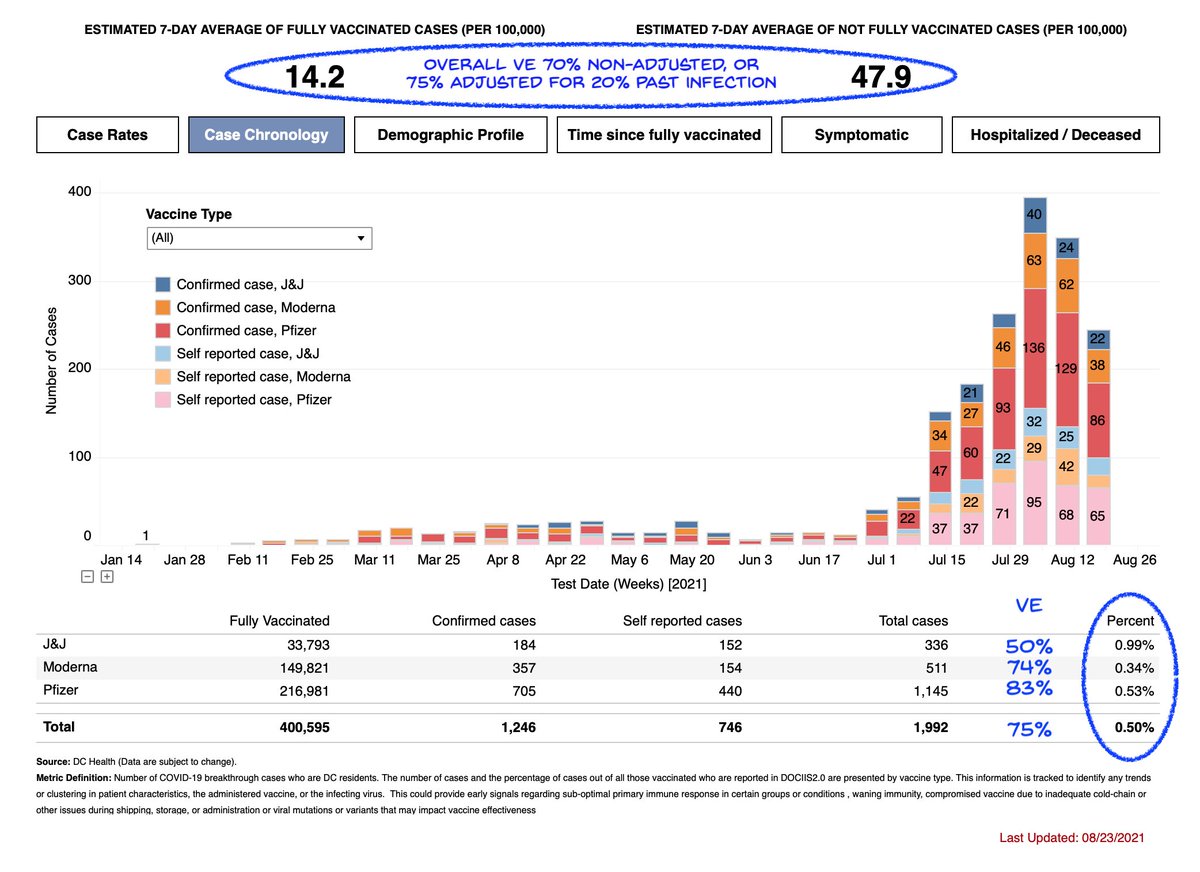
Based on DC numbers, I had estimated J&J effectiveness of 50%.
So today's unofficial announcement from South Africa matches real-world data from the US as well.
So today's unofficial announcement from South Africa matches real-world data from the US as well.
https://twitter.com/michaelzlin/status/1432121982397599745
It is now clear that J&J has worse efficacy on Delta vs 2 doses of RNA vaccines, at the same time post vaccine, for both infection (50 vs >80%) and hospitalizations (71 vs >90%).
I hope the issue can get officially acknowledged soon.
I hope the issue can get officially acknowledged soon.
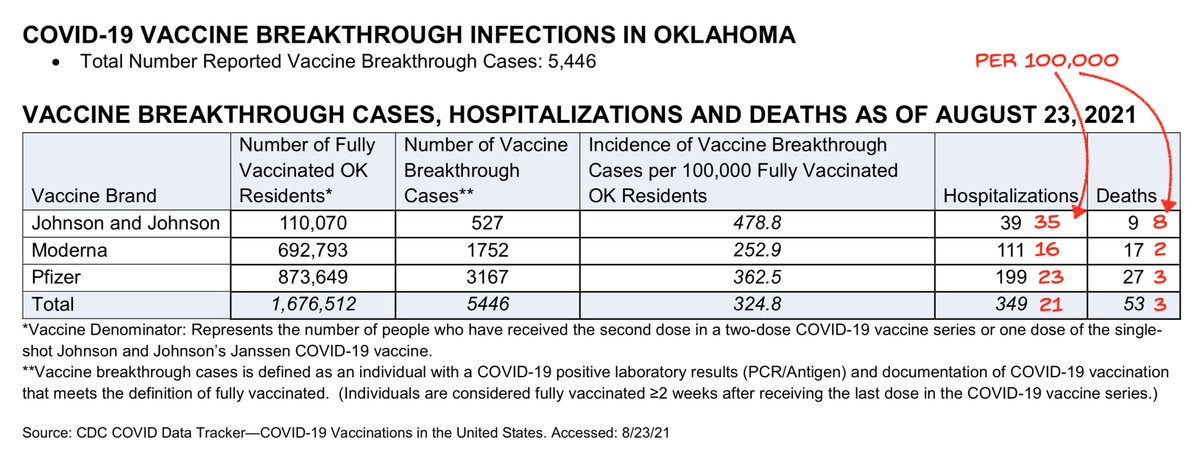
Colorado figures match DC and South Africa.
Relative protection against disease roughly 80/70/50% Moderna/Pfizer/J&J
Thanks @CescaRose80
@apoorva_nyc, you may be interested
Relative protection against disease roughly 80/70/50% Moderna/Pfizer/J&J
Thanks @CescaRose80
@apoorva_nyc, you may be interested

More J&J in the news today. But you can't have a J&J article without a someone making an incorrect and poorly informed point, apparently.
washingtonpost.com/health/2021/09…
washingtonpost.com/health/2021/09…
"“I think we should have some humility in that nobody really knows” if a combination approach helps, she said."
We know from Stephensen and Barouch study, and the 90,000 people who did a RNA boost after J&J can be studied. More info at our op-ed at nytimes.com/2021/08/29/opi…
We know from Stephensen and Barouch study, and the 90,000 people who did a RNA boost after J&J can be studied. More info at our op-ed at nytimes.com/2021/08/29/opi…

"“If one had shown more risk than the other, they would have said so,” she said."
One has – see above. And they haven't said so.
Even without the evidence above, we've learned that that CDC prefers not to say some things. So not sure anyone should find CDC silence reassuring.
One has – see above. And they haven't said so.
Even without the evidence above, we've learned that that CDC prefers not to say some things. So not sure anyone should find CDC silence reassuring.

• • •
Missing some Tweet in this thread? You can try to
force a refresh