And there you go
New CDC stuy shows best estimate of J&J efficacy vs Delta hospitalization is 60%
This is worse than 80% for Pfizer and 95% for Moderna
cdc.gov/mmwr/volumes/7…
New CDC stuy shows best estimate of J&J efficacy vs Delta hospitalization is 60%
This is worse than 80% for Pfizer and 95% for Moderna
cdc.gov/mmwr/volumes/7…

And before anyone complains the CIs are overlapping, that's a sophomoric complaint. Remember that's the 95% CI, and 95% is an arbitrary bar. Also there are other data that show J&J's lower effectiveness on hospitalization, such as below (pre-Delta)
https://twitter.com/EricTopol/status/1435722838284013570
Or the finding that J&J is only 71% effective vs hospitalization due to Delta, from South Africa
https://twitter.com/profshanecrotty/status/1423684812309626889
BTW if you're going to say there's a chance the J&J vaccine could be up to 77% effective (top end of 95% CI, so there's only a 2.5% chance of that) and that overlaps with the 73% low end of the Pfizer effectiveness (again only a 2.5% chance of that), then...
you also have to say there's an equally likely chance that the J&J vaccine is as low as 31% effective vs Pfizer's 85%. So yes, you can pick ends of a 95% CI interval to say there's a small likelihood of actual no difference, but you're most likely to be wrong.
So yes there is very much such a thing as improper interpretation of statistics, which is the science of quantifying uncertainty. You can try to impose edge interpretations onto your statistical results, but doing so repeatedly renders statistics meaningless.
In any case, the difference between J&J and Moderna effectiveness is statistically significant beyond 95% as well. This is very much a clear case.
Back to the topic at hand, this is very reassuring that @CDCgov itself has data showing lower efficacy of the J&J vaccine, on top of 2 other studies showing ~70% efficacy vs hospitalizations of the J&J vaccine.
I am optimistic that something can now be done for #JnJers.
I am optimistic that something can now be done for #JnJers.
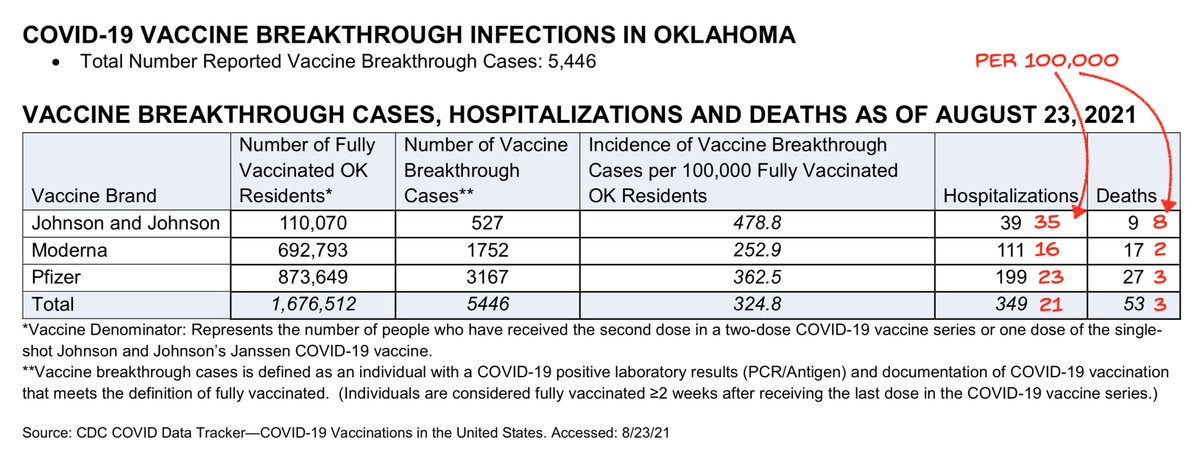
And if you count OK numbers as more evidence, I had seen earlier that J&J protection vs hospitalization also lagged the other vaccines, with statistical significance vs Delta (scroll down in linked thread)
https://twitter.com/michaelzlin/status/1430794839415660544
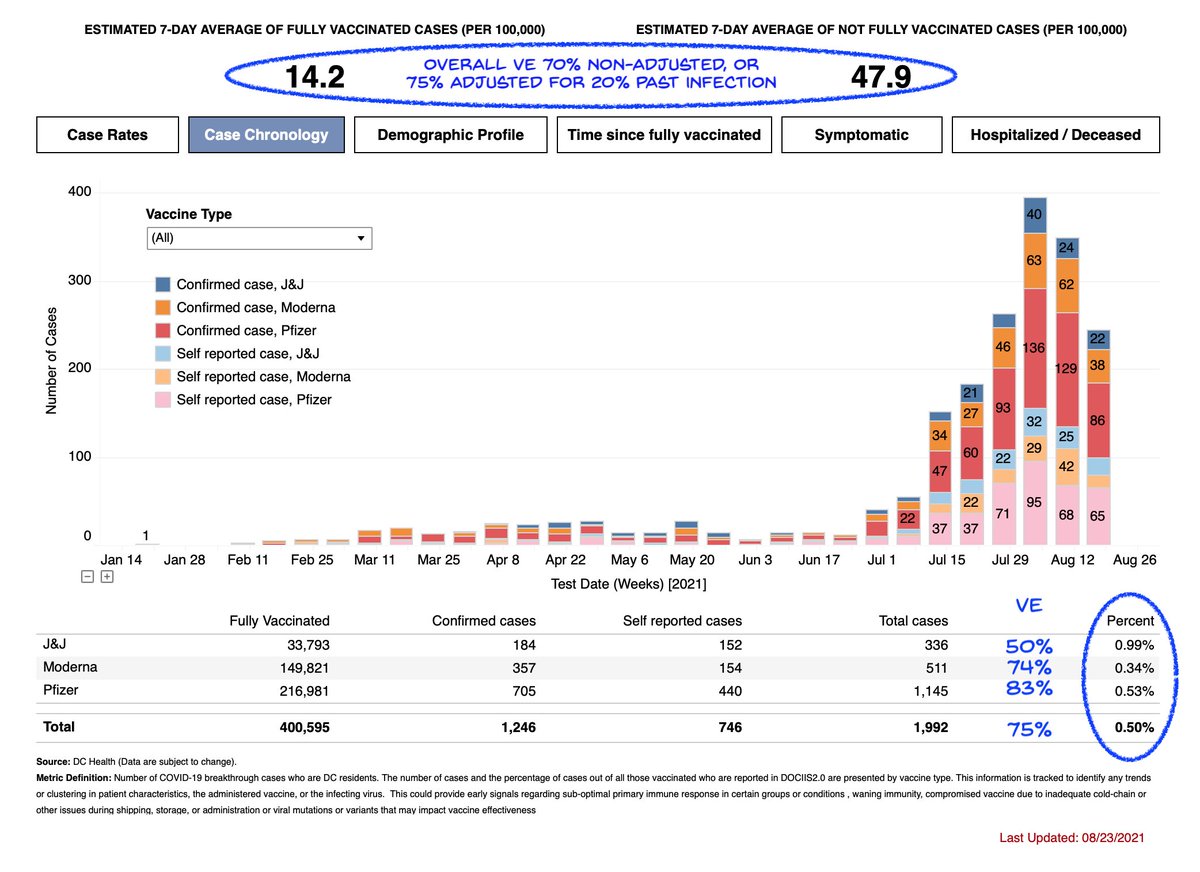
The above numbers just for hospitalization. If you look at J&J protection vs cases from Delta, it looks even worse at ~50%, both in my analysis of DC breakthroughs below
https://twitter.com/michaelzlin/status/1432121982397599745
J&J protection from Delta cases was also 50% in reported CO breakthroughs (CO data was included in today's CDC report on hospitalizations)
https://twitter.com/michaelzlin/status/1435763429629837317
And J&J protection from Delta infection was also "about half" in unofficial comments from the South Africa trial
fortune.com/2021/09/07/joh…
fortune.com/2021/09/07/joh…
Such low numbers — 70% protection from hospitalization and 50% protection from cases — have been stated by health officials to be unacceptable for RNA vaccinees, which is why boosters are planned before their immunity wanes to that point.
They are already what #JnJers suffer.
They are already what #JnJers suffer.
Thus it's time for #JnJers to get boosters
But for J&J to report their 2-dose trial and FDA to evaluate it will take months, and J&J boost may not be as effective or safe as a RNA boost.
Thus @CDCDirector should allow a Pfizer boost to be prescribed off-label, as the UK allows.
But for J&J to report their 2-dose trial and FDA to evaluate it will take months, and J&J boost may not be as effective or safe as a RNA boost.
Thus @CDCDirector should allow a Pfizer boost to be prescribed off-label, as the UK allows.
h/t @rmalhotraphd
• • •
Missing some Tweet in this thread? You can try to
force a refresh