In our @nytimes opinion piece, Dr. Paul Sax (Brigham and Women's Hospital) and I present why the data support RNA boosters for immunocompromised J&J patients, now possible off-label.
And when boosters kick in for everyone, we hope #JnJers are given good options at the same time.
And when boosters kick in for everyone, we hope #JnJers are given good options at the same time.
https://twitter.com/nytopinion/status/1432032937399230470
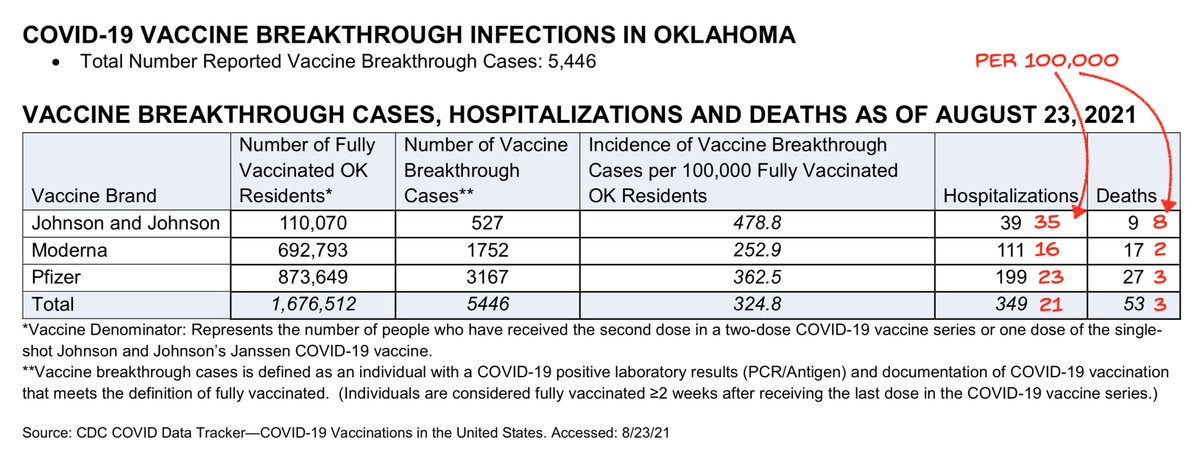
@nytimes From available breakthrough numbers, J&J has consistently shown higher rates than the other two vaccines, as I discuss in the thread below.
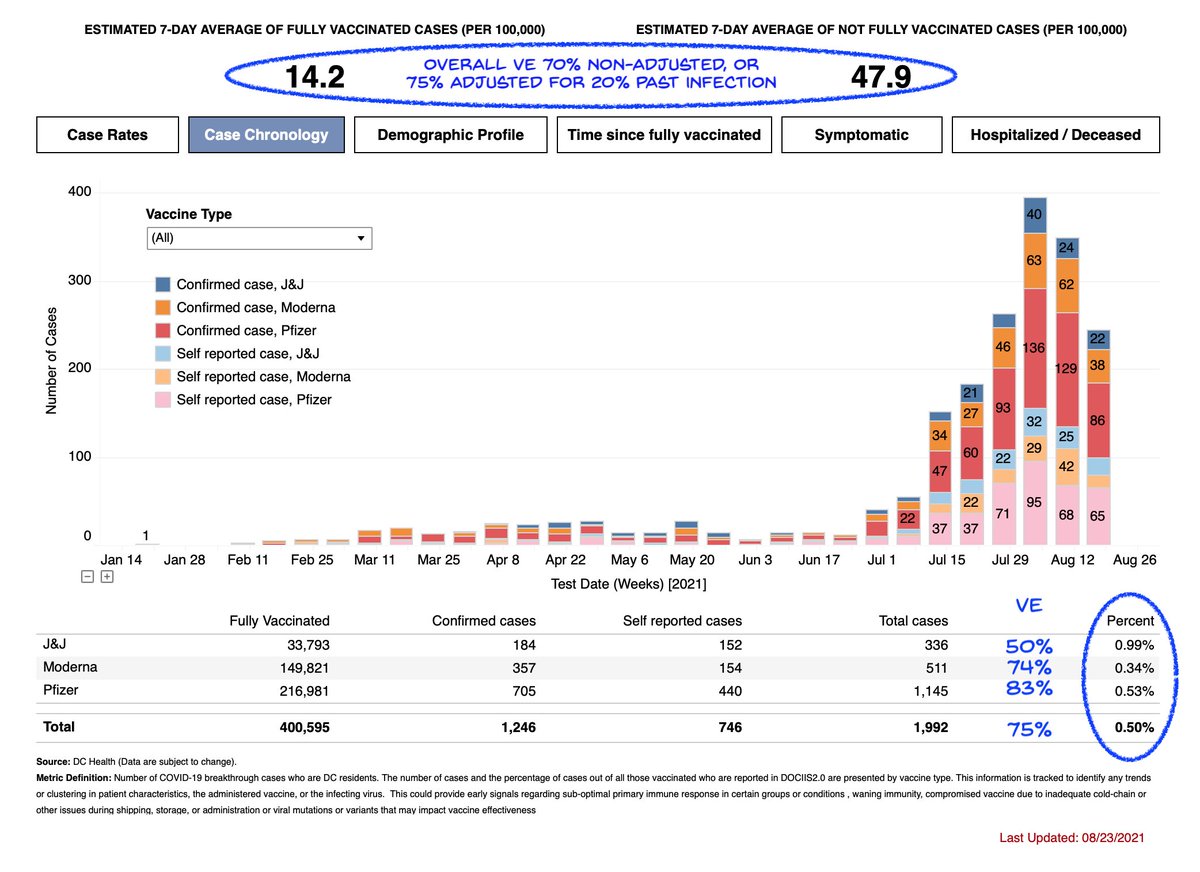
This week's DC numbers suggest in that population J&J vaccine efficacy against infection is about 50%
This week's DC numbers suggest in that population J&J vaccine efficacy against infection is about 50%
https://twitter.com/michaelzlin/status/1432101154519801858
And that's why we think the data we have sitting in databases on J&J VE need to be openly discussed, and why boosters for #JnJers are needed at least at the same time as boosters for everyone else.
Iceland had both Pfizer and J&J vaccines. They have offered Pfizer boosters to all who have received J&J. Iceland has been exemplary in how they have addressed COVID-19, very data-driven.
icelandreview.com/politics/schoo…
icelandreview.com/politics/schoo…
If US always waits for empirical data rather than trusting science and mechanism, we will always lag behind countries like Iceland that are able to weigh all factors. And lagging in a fast-moving epidemic means people will suffer unnecessarily.
Iceland actually explains in detail the reason to give boosters to J&J recipients first, and why the boosters should be RNA and not another J&J. It is a good example of being *both* data- and mechanism-driven.
landlaeknir.is/um-embaettid/g…
landlaeknir.is/um-embaettid/g…
I'll just go through it word for word as it's very informative.
"The first group that the Chief Epidemiologist recommends being offered a booster vaccination is individuals without history of COVID-19/antibodies who were vaccinated with the Janssen vaccine.
"The first group that the Chief Epidemiologist recommends being offered a booster vaccination is individuals without history of COVID-19/antibodies who were vaccinated with the Janssen vaccine.
This group will be offered one dose of Pfizer/BioNTech or Moderna vaccine at least 8 weeks after being vaccinated with the Janssen vaccine. The arguments behind this recommendation are as follows:
•Protection after a single dose of Janssen vaccine is similar to protection after a single dose of other COVID-19 vaccines used in Iceland, about 66% against 74–80% protection against infection from the original Wuhan variant of the virus,
but Janssen has a marketing authorisation as a single dose vaccine and was therefore used as such. [meaning: it wasn't great but that's how it was authorized]
•The protection from 2 doses of vaccines from Pfizer/BioNTech, Moderna or AstraZeneca is better than the protection of 1 dose. [meaning: we expect 2 doses will be better because that's how every other vaccine works]
The manufacturer of Janssen has tested a two-dose schedule in phase 1 & 2 trials using an 8-week interval. A second dose stimulated antibody response, but studies of the effects on the likelihood of infection are lacking. [meaning: recent J&J lab studies not very informative]
Longer-term studies of primary vaccination with 2-dose vaccines for COVID-19 (Pfizer and AstraZeneca vaccines) have shown that the response is no worse and even better if the period between the first dose and the second dose is longer than originally planned by the manufacturers.
•Other vaccines based on the same technology usually do not use the same vaccine for the booster dose as for the first dose (Janssen’s Ebola vaccine8 and the Sputnik V9 COVID-19 vaccine). [meaning: it's thought to be safer and more efficacious not to use the same Ad twice]
•Studies on AstraZeneca’s COVID-19 vaccine’s stimulation, which is based on technology comparable to Janssen’s along [must mean boosting with] mRNA vaccines such as the Pfizer/BioNTech and Moderna vaccines, have shown very good stimulation in terms of antibody production.
•Very little information is available on the efficacy of the Janssen vaccine against the Delta variant, which has now gained the upper hand in Iceland as well as elsewhere, but information is available on the excellent efficacy of the Pfizer/BioNTech vaccine against this variant
•So far, during this 4th wave in Iceland, about 40% of those vaccinated who have been diagnosed with SARS-CoV-2 have been vaccinated with the Janssen vaccine. However, this should be interpreted with caution.
The age group that is now most prominent in terms of infection, 18–49 years old, was largely vaccinated with Janssen, around 36%, but 21% of all vaccinated received the Janssen vaccine.
•Many kindergarten and primary school teachers were vaccinated with the Janssen vaccine. As the Delta variant appears to have become widespread in the community, it is urgent to strengthen the protection of these professions before the start of the school year,
especially given that children in kindergarten and primary schools have not been vaccinated in general vaccinations yet."
All the logic above applies to the US as well
h/t @NateSilver538
All the logic above applies to the US as well
h/t @NateSilver538
• • •
Missing some Tweet in this thread? You can try to
force a refresh