NEW: [Thread] 1. #JnJ 2day announced (via a press release: bit.ly/3AFFCu0):
1. JnJ's #COVID19 jab = more effective when given as a double shot, 56 days - 6 mnths apart (vs. just 1 shot)
2. The efficacy of 1 shot doesn't wane, but a booster shot makes it more effective.
1. JnJ's #COVID19 jab = more effective when given as a double shot, 56 days - 6 mnths apart (vs. just 1 shot)
2. The efficacy of 1 shot doesn't wane, but a booster shot makes it more effective.

2. When a 2nd #JnJ shot is given 56 days after the 1st one, the 2-dose combo provides:
* 100% protection @ severe #COVID19 14 days after the 2nd shot
* 75% protection @ symptomatic (moderate to severe/critical) COVID19 in the countries where the jab was tested
* 100% protection @ severe #COVID19 14 days after the 2nd shot
* 75% protection @ symptomatic (moderate to severe/critical) COVID19 in the countries where the jab was tested

3. When a 2nd/booster shot of the #JnJ #COVID19 jab was given 56 days after the first shot, trial participants' antibody levels rose to 4-6x times higher than after just one shot. 
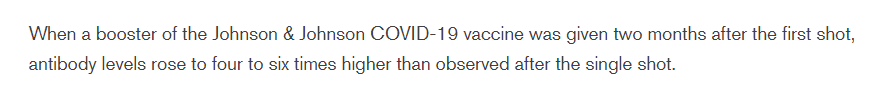
4. But when a 2nd/booster shot was given 6 months after the 1st shot, antibody levels increased even more than when it was given 56 days apart:
* 56 days interval: 4-6-fold increase in antibodies
* 6 months interval: 12-fold increase in antibodies (4 weeks after the 2nd shot)
* 56 days interval: 4-6-fold increase in antibodies
* 6 months interval: 12-fold increase in antibodies (4 weeks after the 2nd shot)

5. Does 1 #JnJ shot still work? YES.
A JnJ preprint study, using real-life data (claims from an insurance company) to analyse the efficacy of 1 dose shows:
* 81% effectiveness @ #COVID hospitalisations
* 79% effectiveness @ #COVID19 infection
Study: medrxiv.org/content/10.110…
A JnJ preprint study, using real-life data (claims from an insurance company) to analyse the efficacy of 1 dose shows:
* 81% effectiveness @ #COVID hospitalisations
* 79% effectiveness @ #COVID19 infection
Study: medrxiv.org/content/10.110…

6. What does this all mean? Statnews says:
"The data appears to set the stage for a discussion over whether the #JnJ vaccine should be thought of as a 2-dose regimen, with the doses given 6 months apart."
Statnews story: bit.ly/3hP419e
"The data appears to set the stage for a discussion over whether the #JnJ vaccine should be thought of as a 2-dose regimen, with the doses given 6 months apart."
Statnews story: bit.ly/3hP419e
7. But for #JnJ to be considered as 2 doses, A LOT more needs to happen:
* Regulators such as the FDA need to convene open meetings
* Much more detailed data will need to be made public/published (and peer reviewed)
* Regulators such as the FDA need to convene open meetings
* Much more detailed data will need to be made public/published (and peer reviewed)
8. So what now? #JnJ says it has/will submit data to:
* The US Food and Drug Administration (FDA) and other regulators
* The WHO and National Immunization Technical Advisory Groups
* The US Food and Drug Administration (FDA) and other regulators
* The WHO and National Immunization Technical Advisory Groups

9. What will happen in SA? We will continue to use one dose of #JnJ until data has been submitted to Sahpra (our regulator) and reviewed by the body. If Sahpra approves the data, the health dpt can then decide if two doses are necessary or not.
• • •
Missing some Tweet in this thread? You can try to
force a refresh