
It's #WorldPolioDay!
5⃣ of the 6⃣ WHO regions – representing over 9⃣0⃣% of the 🌎🌍🌏 population – are certified free of wild polio.
Join us in celebrating the progress we have made, & the work we must do to #EndPolio everywhere.
👉 bit.ly/2ZcYsuI
5⃣ of the 6⃣ WHO regions – representing over 9⃣0⃣% of the 🌎🌍🌏 population – are certified free of wild polio.
Join us in celebrating the progress we have made, & the work we must do to #EndPolio everywhere.
👉 bit.ly/2ZcYsuI
It's #WorldPolioDay
Polio cases are down 99.9% since 1988, but the #COVID19 pandemic has set back vaccinations and placed this incredible progress at risk. Let's strengthen our efforts to #EndPolio and ensure that progress against this virus is not lost.
Polio cases are down 99.9% since 1988, but the #COVID19 pandemic has set back vaccinations and placed this incredible progress at risk. Let's strengthen our efforts to #EndPolio and ensure that progress against this virus is not lost.
In 2020, after 4⃣ years without a case, @WHOAFRO has been certified as wild polio-free!
On this #WorldPolioDay, take a moment to learn about remarkable success against wild #polio in Africa.
👉bit.ly/3jjNV5j
On this #WorldPolioDay, take a moment to learn about remarkable success against wild #polio in Africa.
👉bit.ly/3jjNV5j
#Polio is a highly infectious viral disease.
It’s transmitted from person-to-person mainly through the faecal-oral route or via contaminated water or food. It can invade the nervous system & cause paralysis.
👉 bit.ly/2ZcYsuI
It’s transmitted from person-to-person mainly through the faecal-oral route or via contaminated water or food. It can invade the nervous system & cause paralysis.
👉 bit.ly/2ZcYsuI
It's #WorldPolioDay
Polio mainly affects children under 5 years of age.
There is no cure for #polio, but it can be prevented. Polio vaccine, given multiple times, can protect a child for life.
👉 bit.ly/2ZcYsuI
Polio mainly affects children under 5 years of age.
There is no cure for #polio, but it can be prevented. Polio vaccine, given multiple times, can protect a child for life.
👉 bit.ly/2ZcYsuI
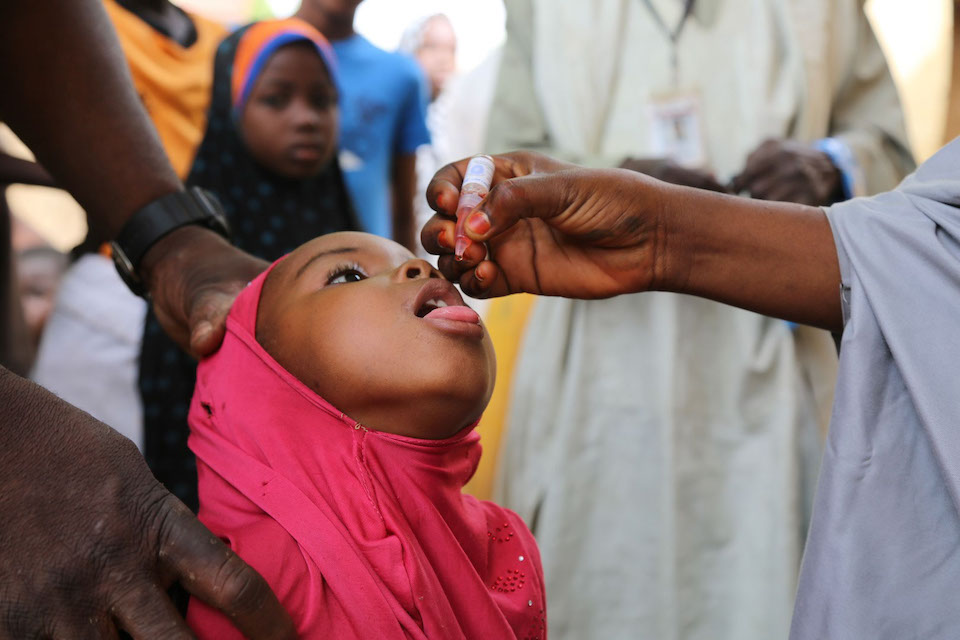
It's #WorldPolioDay
There are 3⃣ strains of wild poliovirus.
Type 2 and type 3 have officially been certified as globally eradicated.
Wild poliovirus type 1 still affects two countries:
🔸 Pakistan
🔸 Afghanistan
👉 bit.ly/2ZcYsuI
There are 3⃣ strains of wild poliovirus.
Type 2 and type 3 have officially been certified as globally eradicated.
Wild poliovirus type 1 still affects two countries:
🔸 Pakistan
🔸 Afghanistan
👉 bit.ly/2ZcYsuI

Two national #polio campaigns were recently announced in Afghanistan.
It is critical that health workers reach all children with vaccines to #EndPolio.
It is critical that health workers reach all children with vaccines to #EndPolio.
https://twitter.com/WHOAfghanistan/status/1449955985439797248?s=20
The 8 November #polio campaign in Afghanistan - the first national house-to-house campaign in over 3 years - will be followed by a second campaign, to be synchronized with Pakistan in December.
👉 bit.ly/3j79ybZ
👉 bit.ly/3j79ybZ

In many communities around the 🌍, women health workers help navigate social, cultural religious and political contexts to engage with communities. The #polio program’s strategy will continue supporting the women who are central in the fight to #EndPolio. 

It's #WorldPolioDay
For decades, frontline vaccinators have worked to #EndPolio.
Join us in thanking them as they continue to vaccinate children, save lives, and combat this terrible disease.
For decades, frontline vaccinators have worked to #EndPolio.
Join us in thanking them as they continue to vaccinate children, save lives, and combat this terrible disease.
It's #WorldPolioDay
#Polio workers leave a purple mark on the pinky finger of children they vaccinate. They also leave their mark on the 🌍 by providing other needed services like clean water, nutrition supplements, bed nets and much more. #EndPolio
📸@UNICEF
#Polio workers leave a purple mark on the pinky finger of children they vaccinate. They also leave their mark on the 🌍 by providing other needed services like clean water, nutrition supplements, bed nets and much more. #EndPolio
📸@UNICEF

Alongside their work to #EndPolio, polio workers:
✅Help distribute #COVID19 vaccines
✅Conduct surveillance for multiple diseases
✅Respond to health emergencies
In countries that are #polio-free, we help people repurpose these skills to improve a range of health outcomes!
✅Help distribute #COVID19 vaccines
✅Conduct surveillance for multiple diseases
✅Respond to health emergencies
In countries that are #polio-free, we help people repurpose these skills to improve a range of health outcomes!

The work of the #polio program doesn’t stop with a vaccine. The infrastructure created to #EndPolio around the world is being used every day to:
🏙️ promote cleaner, safer communities
🛡️ fight other diseases
🔨 build a healthier future to achieve #HealthForAll
🏙️ promote cleaner, safer communities
🛡️ fight other diseases
🔨 build a healthier future to achieve #HealthForAll
#Polio eradication takes commitment from:
👥vaccinators
👥activists
👥community mobilizers
👥teachers
👥nurses
👥imams
👥priests
👥community elders
👥local leaders
👥shopkeepers
👥parents
👥and countless others
We are stronger together.
Together, we can #EndPolio 💪
👥vaccinators
👥activists
👥community mobilizers
👥teachers
👥nurses
👥imams
👥priests
👥community elders
👥local leaders
👥shopkeepers
👥parents
👥and countless others
We are stronger together.
Together, we can #EndPolio 💪

It's #WorldPolioDay
We have come so far in the fight against polio thanks to the leadership of countless #polio champions. A polio-free 🌎🌍🌏 is possible, but only with the continued commitment from all levels of government.
Let’s #EndPolio!
We have come so far in the fight against polio thanks to the leadership of countless #polio champions. A polio-free 🌎🌍🌏 is possible, but only with the continued commitment from all levels of government.
Let’s #EndPolio!

• • •
Missing some Tweet in this thread? You can try to
force a refresh










