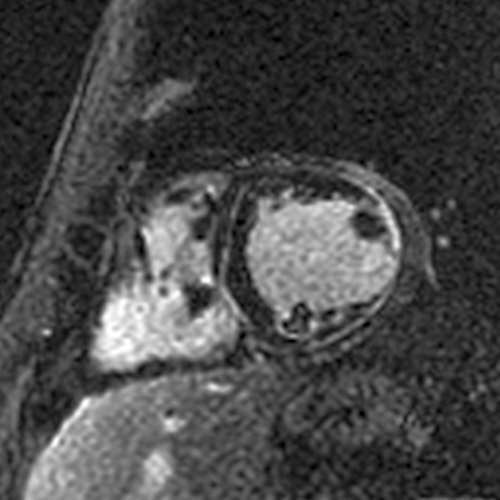
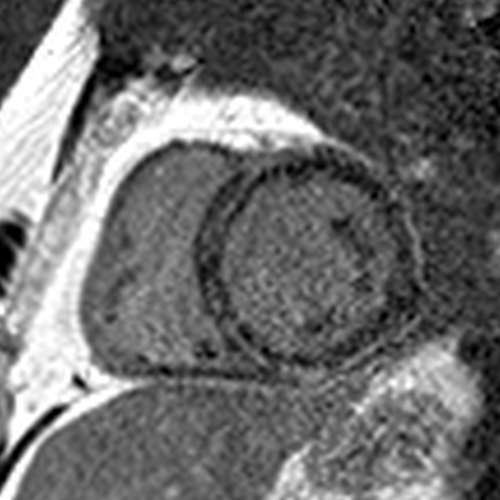
This is an interesting study by @amritlota et al.
“LGE has no prognostic value if LV volumes and EF are normal” is the wrong interpretation, @MAecocardio.
These are my thoughts…
#WhyCMR
“LGE has no prognostic value if LV volumes and EF are normal” is the wrong interpretation, @MAecocardio.
These are my thoughts…
#WhyCMR
https://twitter.com/MAecocardio/status/1469990529920290817
First, was the LGE in this study an artifact ("overcalling")?
No, because:
- This paper comes from pioneers and some of the biggest names in CMR
- Our anecdotal experience matches these findings
- The images in the paper very clearly demonstrate LGE.


No, because:
- This paper comes from pioneers and some of the biggest names in CMR
- Our anecdotal experience matches these findings
- The images in the paper very clearly demonstrate LGE.
https://twitter.com/VDelgadoGarcia/status/1469992047281156103?s=20
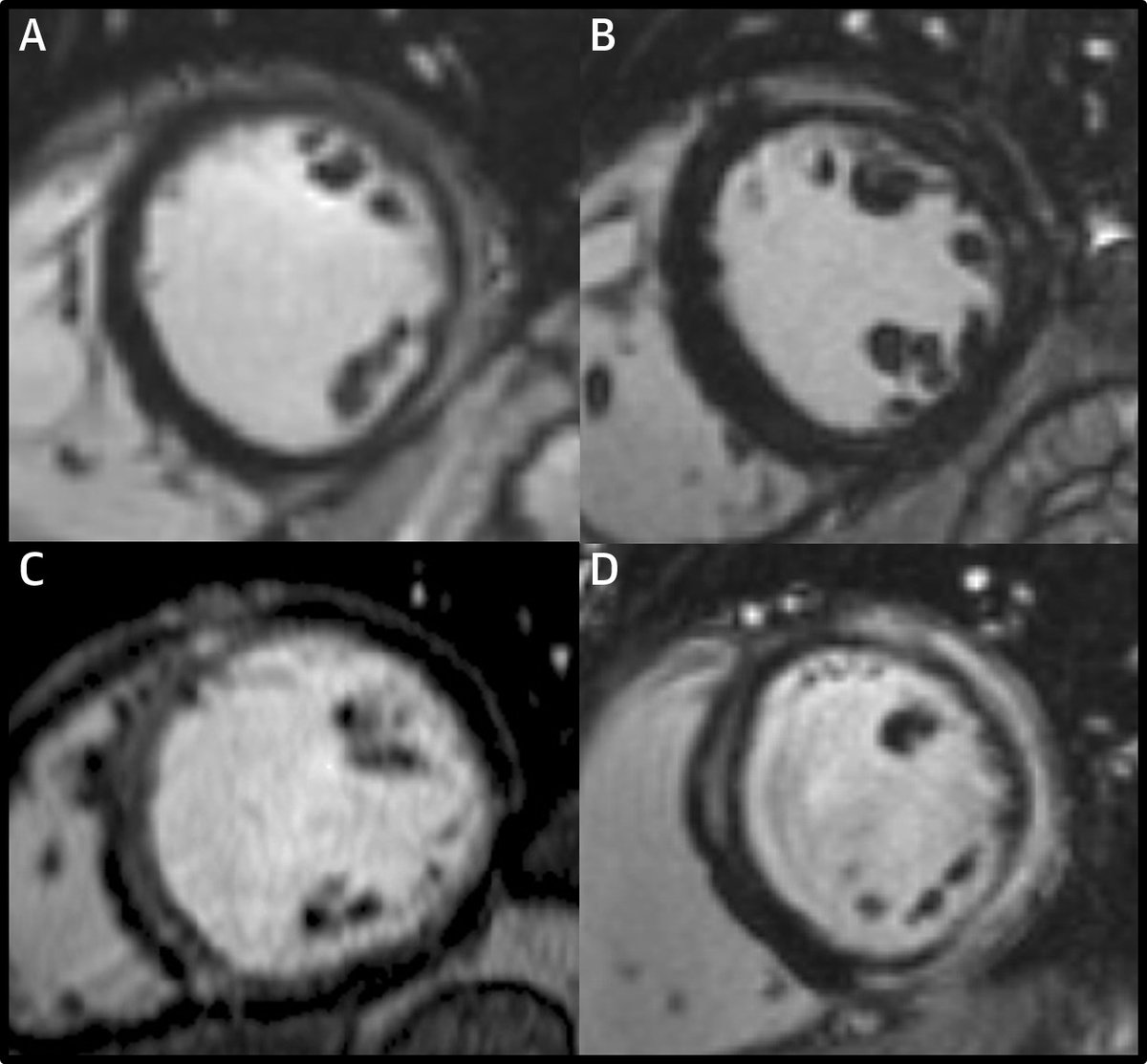

So why was LGE associated with a very low risk of SCD during follow-up in this study?
LGE is always bad at a group/population level compared to no LGE. Is it bad for every person? Like any risk marker, no.
LGE is always bad at a group/population level compared to no LGE. Is it bad for every person? Like any risk marker, no.
The most important determinant of the prognostic value of LGE is the cause of the LGE. Why does the patient have the LGE? What's the cardiomyopathy?
Other than perhaps LGE limited to the insertion sites, LGE is always a pathologic finding even if the volumes and EF are normal.
Other than perhaps LGE limited to the insertion sites, LGE is always a pathologic finding even if the volumes and EF are normal.
But the prognostic value of LGE is not the same across all cardiomyopathies.
This is one reason why simply using the presence of LGE (without considering the etiology) is not a great way to identify the risk of SCD, as we discussed in this editorial:
sciencedirect.com/science/articl…
This is one reason why simply using the presence of LGE (without considering the etiology) is not a great way to identify the risk of SCD, as we discussed in this editorial:
sciencedirect.com/science/articl…

Based on the LGE patterns in the images in this paper and our own experience, my guess is that a significant proportion of the LGE in this study is due to genetic cardiomyopathies.
A family history of SCD is not a great screening tool for genetic cardiomyopathies.

A family history of SCD is not a great screening tool for genetic cardiomyopathies.


With that perspective, what this study tells me is that the risk of SCD is very low over a median of 4.3 years in low-risk -- based on normal LV volumes, normal EF, normal LV mass, and a median LGE of 2.25% -- patients with mostly genetic cardiomyopathies. 

Is this surprising? Not really… in recent years, genetic cardiomyopathies are being recognized more and more, and we are also learning that it is not ominous at all.
https://twitter.com/cshenoy3/status/1450869534865477641?s=20
And this is well described in one type of genetic cardiomyopathy - HCM.
In patients deemed to be low risk for SCD in HCM, the risk of SCD is very low despite the presence of LGE in many.
In patients deemed to be low risk for SCD in HCM, the risk of SCD is very low despite the presence of LGE in many.
In this recent HCM paper, only 2 of around 1600 HCM patients deemed to be low risk had SCD in the long term.
jamanetwork.com/journals/jamac…
jamanetwork.com/journals/jamac…
It is important to remember that genetic cardiomyopathies can progress over time. With longer follow-up and larger sample sizes, I anticipate LGE will be associated with SCDs in patients with normal volumes, normal EFs, and a small amount of LGE at baseline/when first assessed.
LGE (which indicates irreversible myocardial damage) will always have an adverse prognostic impact compared to no LGE, at a group/population level, in adequately powered studies.
On an individual level, the prognostic impact varies and depends primarily on the cause of the LGE.
On an individual level, the prognostic impact varies and depends primarily on the cause of the LGE.
• • •
Missing some Tweet in this thread? You can try to
force a refresh