Today we're thrilled to announce results of our PREVENT-19 Phase 3 pediatric expansion to adolescents (ages 12 through 17). Let's talk about the data and what they mean. Thread: 

Today's results build on findings from the adult main study, investigating the safety & efficacy of NVX-CoV2373 in the United States and Mexico amidst an evolving #COVID19 pandemic: nejm.org/doi/full/10.10… #clinicaltrials
The pediatric expansion randomized adolescents age 12 through 17, in the U.S., in a 2:1 ratio to receive 2x 5μg doses of NVX-CoV2373, our investigational #vaccine candidate, or placebo, 21 days apart. Protocol: novavax.com/sites/default/… 

The adolescent study also included a blinded crossover, where placebo recipients received active vaccine & vice versa, ensuring all participants received NVX-CoV2373 without compromising @FDA-required safety follow-up. 

We'll be talking about the study's 2 key endpoints: 1) Primary Effectiveness Endpoint: the non-inferiority of neutralizing antibody responses vs young adults (18-26) from the adult part of the study;
and 2) Primary Efficacy Endpoint: PCR-confirmed, symptomatic mild, moderate or severe #COVID19 diagnosed ≥7 days after the 2nd vaccine dose.
Demographics of the 2,247 participants were well balanced between vaccine and placebo groups. There were more younger participants as EUA vaccines weren't available for that age group at the time. 

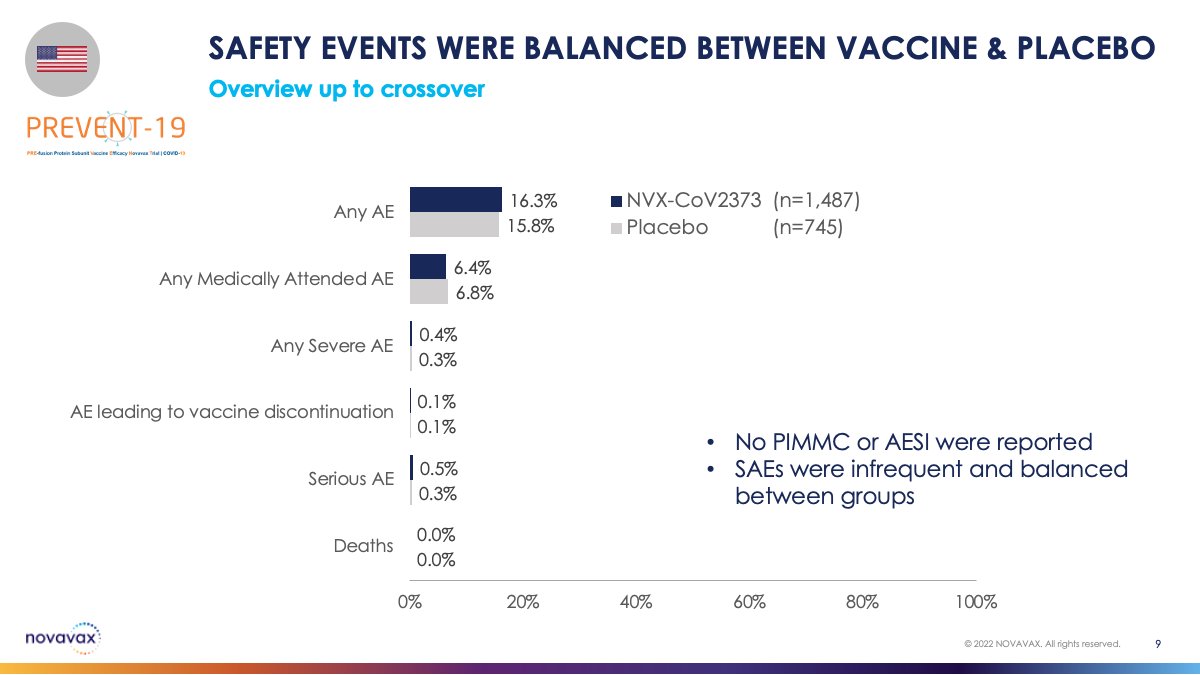
Safety events were balanced between vaccine and placebo groups. Serious Adverse events were infrequent and balanced. No safety signals were observed. 
The vaccine was well tolerated. Local reactogenicity was similar to adults. Pain & tenderness were the most common. Findings were consistent between younger and older adolescents. 

Similar findings were observed for systemic reactogenicity. As with local reactogenicity, there was an increase after the second dose, as expected. 

The vaccine demonstrated its effectiveness in this study: Neutralizing antibody responses were non-inferior to young adults, showing the vaccine performs in adolescents. Licensure-enabling effectivness endpoint met ✅. 

Not only were neutralizing responses non-inferior, they were higher: ~1.5X higher immune responses than in adults in the Phase 3 US/MX trial, which were associated with high levels of efficacy. Seroconversion was ~99%. nejm.org/doi/full/10.10…
Overall, adolescent antibody titers were higher than in adults. Titers were higher in younger than in older adolescents. 

Antibodies showed the ability to identify variants. After 2 doses, 100% had detectable IgG responses against all variants tested, including #Omicron. These responses were 2-3X higher than in adults. 

Those antibodies were functional: they were able to block the binding between the virus and the human ACE2 receptor, for all variants tested. This assay shows the potential to interrupt SARS-CoV-2 mechanism of infection. 

We looked at clinical efficacy against mild, moderate & severe COVID-19, 7 days after 2nd dose. 20 cases were seen: 6 in the vaccine group, 14 in placebo – for a vaccine efficacy of 79.5%. Primary efficacy endpoint met ✅. 

We saw 82% efficacy against Delta VOC, generally matching the overall efficacy observed in the adult study, where we saw efficacy >90%, including against variants, and complete protection against moderate & severe disease. 

Summary: No safety signal was observed. Vaccine was well tolerated. Primary effectiveness and efficacy endpoints were met. Overall efficacy was 79.5% and 82% against Delta VOC. Findings consistent between age groups. 

Novavax looks forward to supplementing our global regulatory findings with results from this pediatric expansion. Additional pediatric studies are expected to start in Q2.
Thank you. Novavax is grateful to the thousands of people around the world who are volunteering for our vaccine studies. We thank the U.S. Government, @CEPIvaccines & other governments for their significant support of our work.
• • •
Missing some Tweet in this thread? You can try to
force a refresh












