Task-free fMRI is governed by high-amplitude BOLD coactivations (termed "CAPs"). But how do these patterns emerge and what do they mean?
New collaborative work conceived by #Diego_Fasoli and #Stefano_Panzeri provides possible explanations 👉tinyurl.com/2p8un5kp
🧵1/n
New collaborative work conceived by #Diego_Fasoli and #Stefano_Panzeri provides possible explanations 👉tinyurl.com/2p8un5kp
🧵1/n

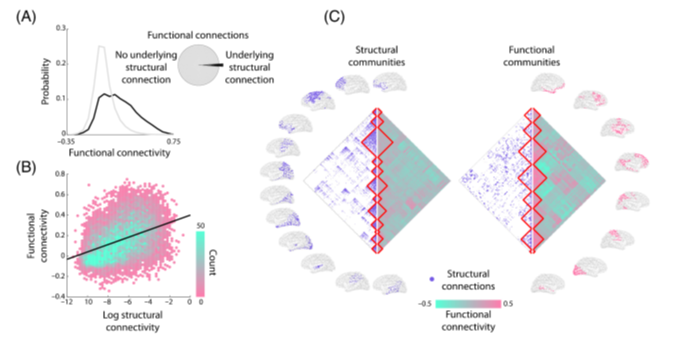
In this work, we developed a large-scale network model of the mouse cortex that combines *directed* axonal connectivity (👉tinyurl.com/mryub799) with *non-linear* firing rate dynamics.
PS: “we” in this thread mostly stands for “Diego & Stefano”
2/n
PS: “we” in this thread mostly stands for “Diego & Stefano”
2/n

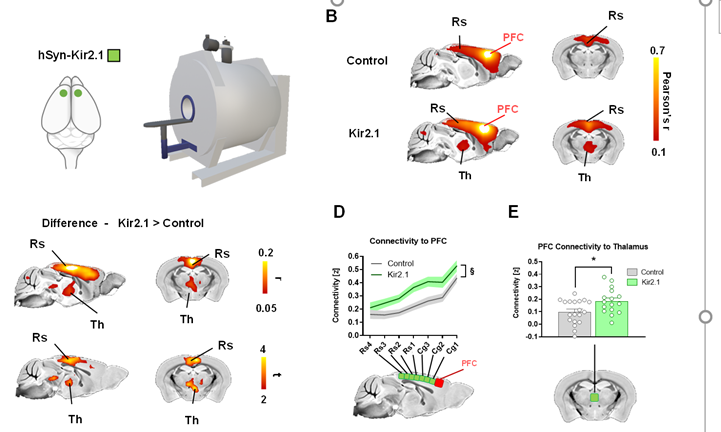
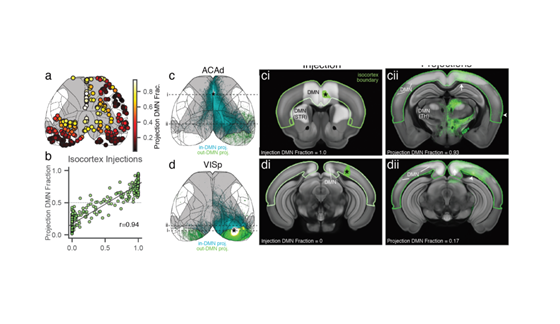
Our model reproduces remarkably well (ρ = 0.55) the topography of empirical 🐁 rsfMRI activity: simulated data show robust inter-hemispheric symmetry, and delineate well-characterized network systems of the mouse such a default-mode network, latero-cortical system etcetera.
3/n
3/n

What about CAPs? Recurrently connected neural networks like the one we used here exhibit attractor dynamics: we thus hypothesized that attractor dynamics may exist in real-world rsfMRI timeseries & that the emergence & features of CAPs may be related to observed attractors
4/n
4/n

We thus analyzed the dynamics of our model numerically and expressed model attractors as patterns of rsfMRI activity. Notably, most attractors we found had symmetric anatomy and emerged in spatially-opposed pairs, recapitulating topography of real-world mouse fMRI CAPs!
5/n
5/n

These results suggest that CAPs are a manifestations of whole-cortex attractor dynamics of the kind we describe in our model!
Remarkably, this rich dynamics emerges only when using a *directed* axonal connectome, and is lost after connectome symmetrization (think of DTI...)
6/n
Remarkably, this rich dynamics emerges only when using a *directed* axonal connectome, and is lost after connectome symmetrization (think of DTI...)
6/n

In the paper we also tested and measured many other features. An interesting finding is for example the ability of our model to predict very well the "fMRI connectivity" anticorrelation observed upon global signal regression..
7/n
7/n

..& the observation that state attractors from the model (as well as those in real fMRI data) encompass transient non-homotopic states (in spite of a highly symmetric connectome) a finding reflecting "Spontaneous Symmetry Breaking" (this one being for hardcore physicists😆)
8/n
8/n

In conclusion, our work adds novel concepts and predictions to existing whole-brain modelling of rsfMRI activity, relating the emergence of high-amplitude cofluctuation patterns (e.g. CAPs) to cortico-cortical attractor dynamics.
9/n
9/n
Very grateful to Diego and Stefano for involving us in this study (and patiently explaining their findings in lay terms to me - hope my summary here makes sense!), @ColettaLudovico @danielgb_87 for help with connectome and CAPs, plus @ERC_Research and all funding agencies
10/10
10/10
• • •
Missing some Tweet in this thread? You can try to
force a refresh