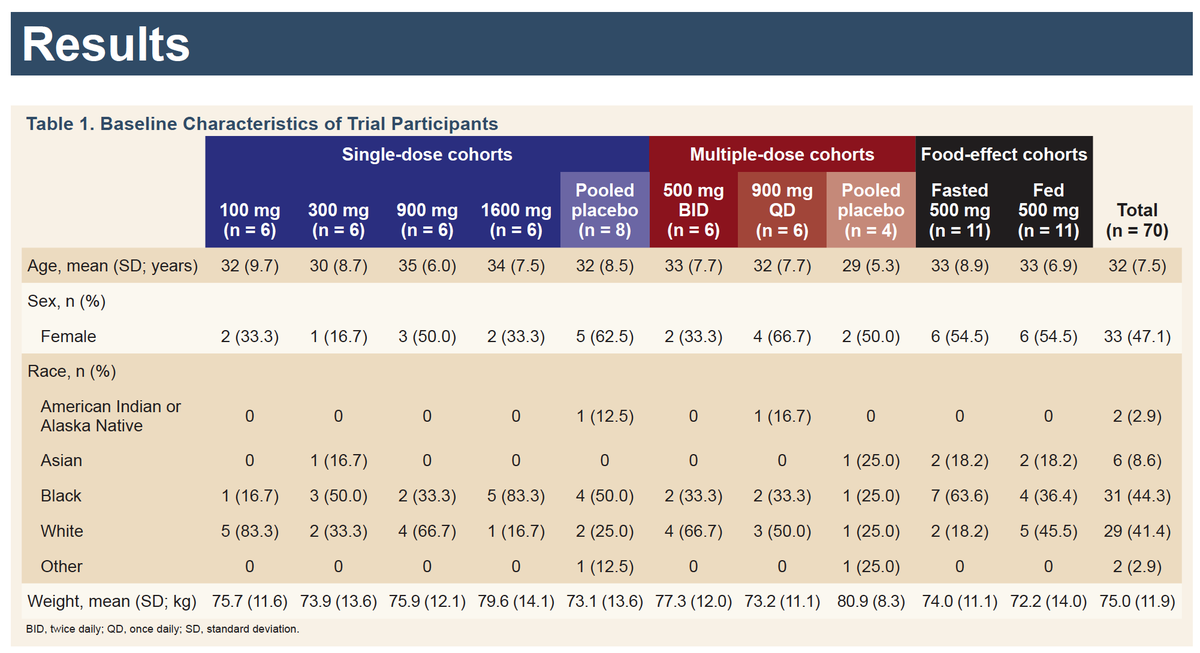
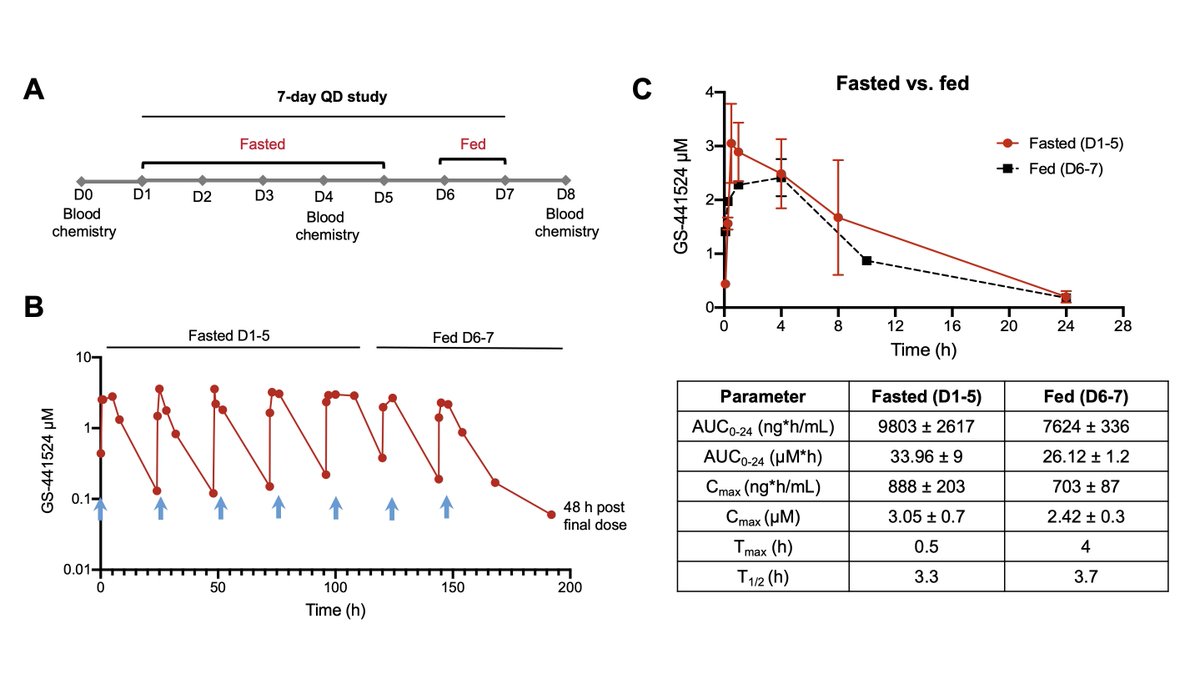
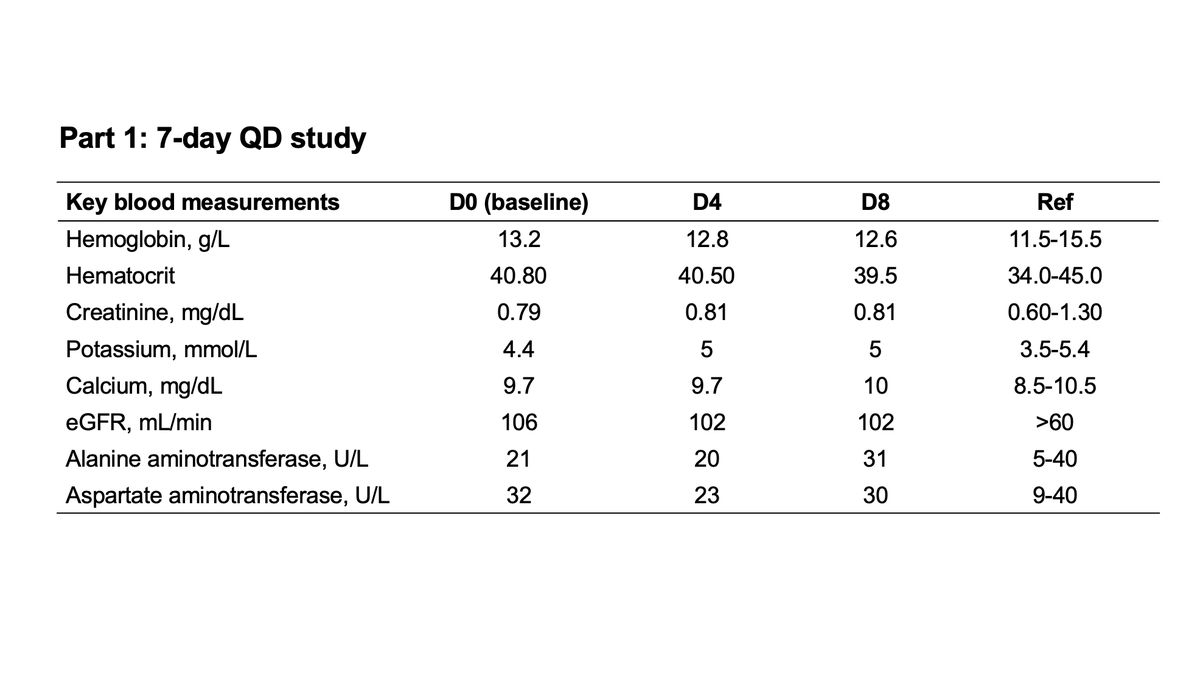
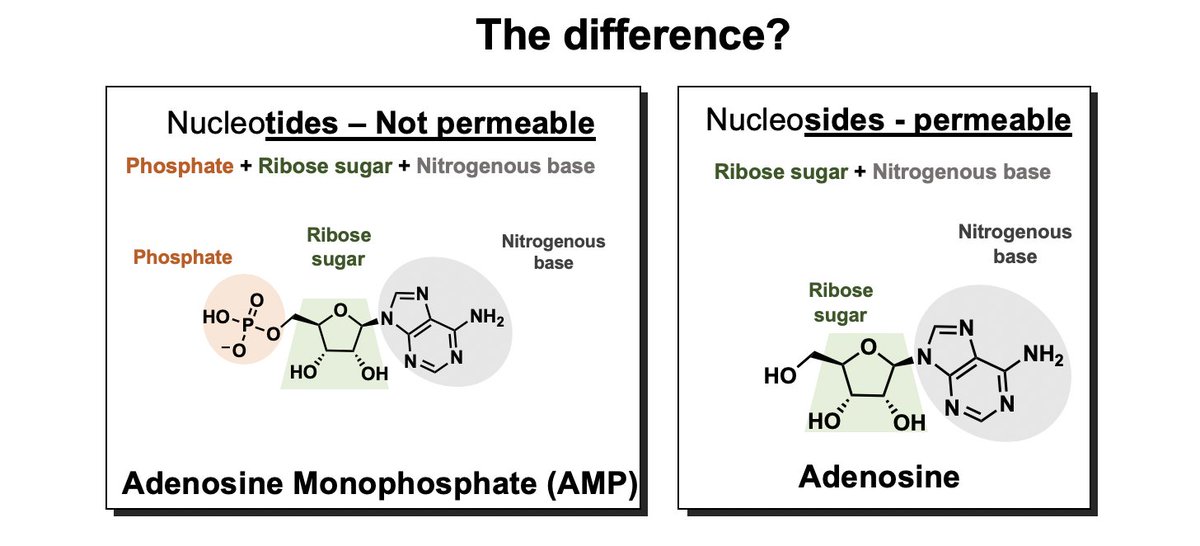
When did @GileadSciences decide that #GS441524 hypothesis was valid? Clues in patent history🔍
While Gilead bashed GS-441524 in 2020, they quietly worked on oral prodrugs
Could we have had an oral COVID pill in late '20/early '21? 💊
I think YES 🧵1/16
worldwide.espacenet.com/patent/search?…
While Gilead bashed GS-441524 in 2020, they quietly worked on oral prodrugs
Could we have had an oral COVID pill in late '20/early '21? 💊
I think YES 🧵1/16
worldwide.espacenet.com/patent/search?…

Patent on oral GS-441524 prodrugs has 1st priority filing on AUGUST 27, 2020. So they did not think the '524 hypothesis was worthless in 2020. 2/16
statnews.com/2020/05/14/gil…
statnews.com/2020/05/14/gil…
Context: @Muller_Lab & I published an Opinion in @statnews urging Gilead to pursue dev of #GS441524 in May 2020 (above)
On June 24, 2020, we first met w Gilead science leadership, who indicated they were not interested. 3/16
medium.com/@victoriacyani…
On June 24, 2020, we first met w Gilead science leadership, who indicated they were not interested. 3/16
medium.com/@victoriacyani…
AUG 27, 2020: 1st priority filing. Includes synthesis & in vitro characterization (RSV, SARS-2) of many ester prodrugs & bioavailability of the tr-ester in cyno.
Mono-iBu ester #obeldesivir (GS-5245) NOT included. 4/16
register.epo.org/documentView?n…


Mono-iBu ester #obeldesivir (GS-5245) NOT included. 4/16
register.epo.org/documentView?n…



These data were later published in J Med Chem in April 2021 (first submitted Jan 2021) in the context of RSV. 5/16
pubs.acs.org/doi/10.1021/ac…
pubs.acs.org/doi/10.1021/ac…

OCT 2020: Many emails later, we convinced Gilead leadership to run a head-to-head study comparing IV #remdesivir with GS-441524 in a monkey model of COVID
'524 showed similar/slightly superior activity, likely facilitating oral '524 program. 6/16
sciencedirect.com/science/articl…

'524 showed similar/slightly superior activity, likely facilitating oral '524 program. 6/16
sciencedirect.com/science/articl…

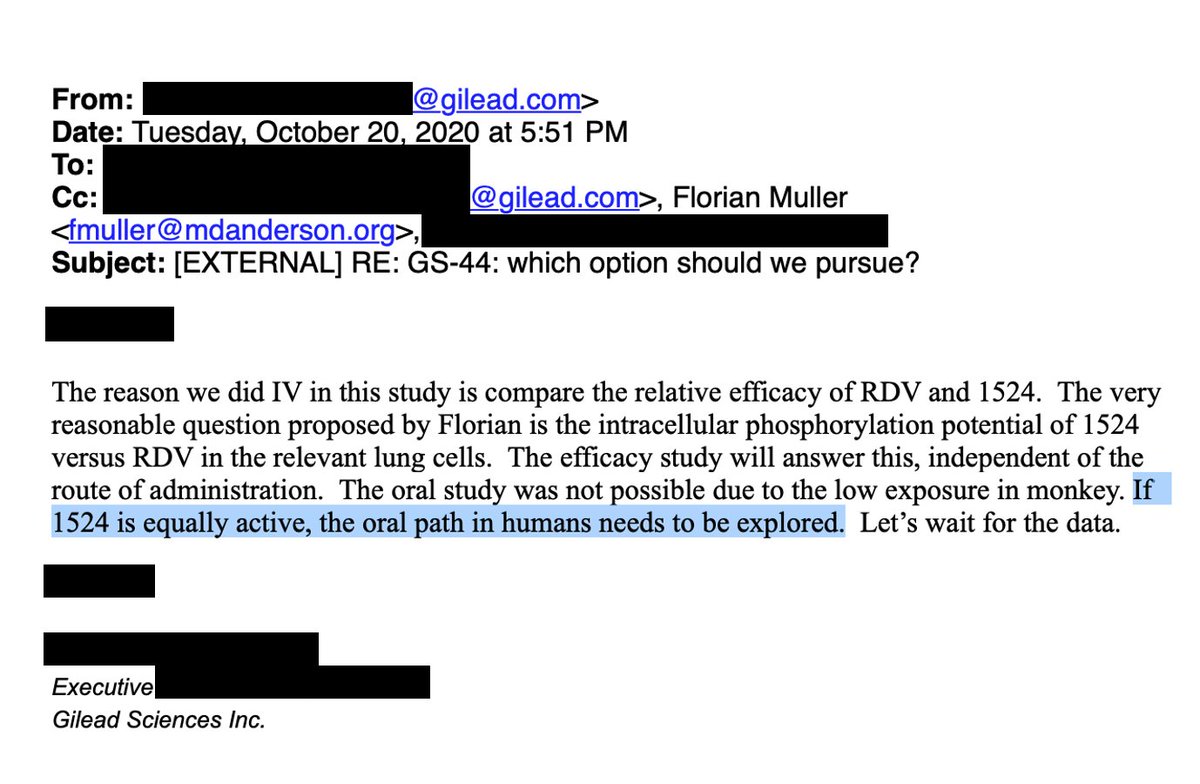
OCT 30, 2020: We met to go over data. They maintained their position on the superiority of RDV. One of them offhandedly said, in ref to oral GS-441524, "we could just put the isobutyric ester on it."
From their summary, they seemed more interested in phosphate prodrugs. 7/16

From their summary, they seemed more interested in phosphate prodrugs. 7/16


MAR 17, 2021: 2nd priority filing. Includes synthesis of & incomplete in vitro characterization of obeldesivir (RSV only, no SARS-2 or CC50 data)
No in vivo PK for obeldesivir. 8/16
register.epo.org/documentView?n…

No in vivo PK for obeldesivir. 8/16
register.epo.org/documentView?n…


JUN 25, 2021: 3rd priority filing. Synthesis of other mono-esters with complete in vitro data in RSV & SARS-2.
F% characterization for tri-ester and obeldesivir across preclinical species.
Efficacy in ferret & mouse models of COVID. 9/16
register.epo.org/documentView?n…

F% characterization for tri-ester and obeldesivir across preclinical species.
Efficacy in ferret & mouse models of COVID. 9/16
register.epo.org/documentView?n…


JUL 30, 2021: Plemper lab posts preprint to Research Square on efficacy of tri-ester in ferret model of COVID. Funded by Gilead.
Gilead states they have no plans to pursue tri-ester
Means they likely decided on obeldesivir in late '20/early '21. 10/16
researchsquare.com/article/rs-662…
Gilead states they have no plans to pursue tri-ester
Means they likely decided on obeldesivir in late '20/early '21. 10/16
researchsquare.com/article/rs-662…
AUG 26, 2021: International filing on GS-441524 ester patent.
Most characterization on obeldesivir (#GS5245), with 2 synthesis routes, in vitro/in vivo efficacy, extensive PK, XRC data. 11/16
Most characterization on obeldesivir (#GS5245), with 2 synthesis routes, in vitro/in vivo efficacy, extensive PK, XRC data. 11/16

SEPT 17, 2021: Sheahan lab posts efficacy of tri-ester (GS-621763) in SARS-2 mouse models to bioRxiv.
GS-621763 shared w Sheahan lab via MTA. 12/16
biorxiv.org/content/10.110…
GS-621763 shared w Sheahan lab via MTA. 12/16
biorxiv.org/content/10.110…

JAN 2022: Gilead states "oral remdesivir" GS-5245 (obeldesivir) in phase 1. 13/16
cnbc.com/video/2022/01/…
cnbc.com/video/2022/01/…
MAR 9, 2022: In NIH Filovirus Workshop, I found that GS-5245 is a GS-441524 prodrug, *NOT* an RDV prodrug, like they seemed to suggest to us back in Oct 2020. 14/16
medium.com/@victoriacyani…
medium.com/@victoriacyani…

NOV 2, 2022: Gilead begins ph3 double-blind RCT with GS-5245 (obeldesivir). 15/16
clinicaltrials.gov/ct2/show/NCT05…
clinicaltrials.gov/ct2/show/NCT05…
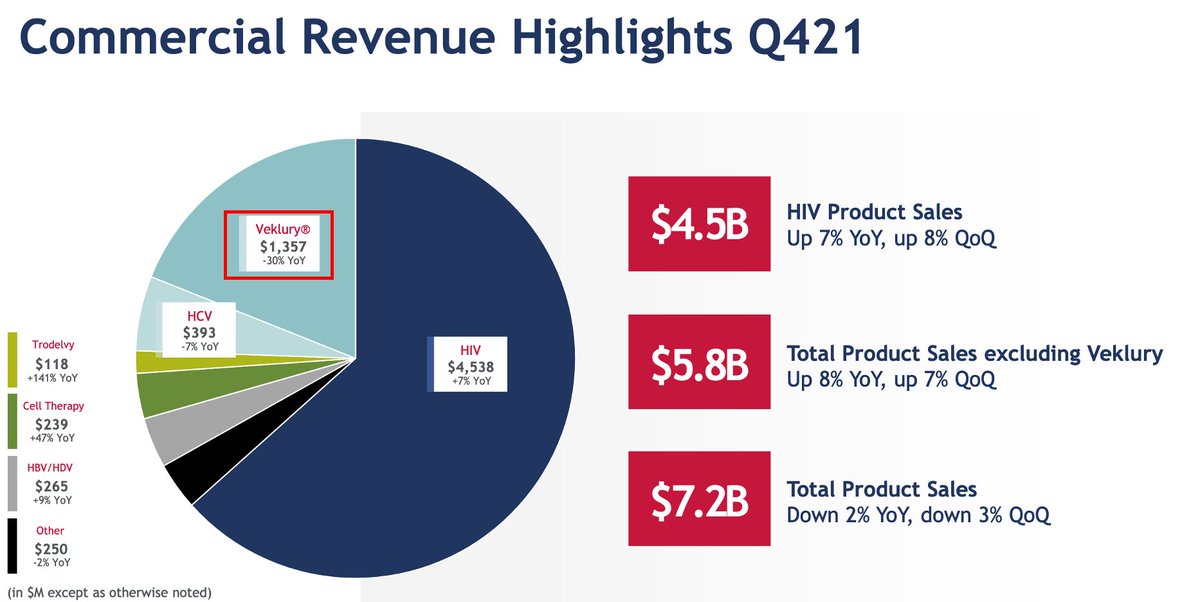
ALL OF THIS TO SAY: while publicly bashing the GS-441524 hypothesis in spring 2020, Gilead was working on oral '524 prodrugs THIS WHOLE TIME!
Could've had an oral COVID tx ~2021!
Milking remdesivir profits, dragging their feet on this project🐄 16/16
s29.q4cdn.com/585078350/file…
Could've had an oral COVID tx ~2021!
Milking remdesivir profits, dragging their feet on this project🐄 16/16
s29.q4cdn.com/585078350/file…
• • •
Missing some Tweet in this thread? You can try to
force a refresh