Sₛᵤₙ = (5,67 × 10⁻⁸ Wm⁻² K⁻⁴)(5800 K)⁴ = 64 × 10⁶ Wm⁻²
Sₛᵤₙ 4π(rₛᵤₙ )² = Sₑₐᵣₜₕ 4π(dₑₐᵣₜₕ)²
Sₑₐᵣₜₕ = Sₛᵤₙ (rₛᵤₙ/dₑₐᵣₜₕ)²
Sₑₐᵣₜₕ = 64 × 10⁶ Wm⁻² (695.510 km/149.600.000 km)²
= 1383,32 Wm⁻²
Sₑₐᵣₜₕ_ₐᵥₑᵣₐ𝓰ₑ = Sₑₐᵣₜₕ /4
Sₑₐᵣₜₕ_ₐᵥₑᵣₐ𝓰ₑ = 1383,32 Wm⁻²/4
= 345,83 Wm⁻²
Tₑₐᵣₜₕ_ₐᵥₑᵣₐ𝓰ₑ = ₄√(Sₑₐᵣₜₕ_ₐᵥₑᵣₐ𝓰ₑ (1 – α )/σ )
Tₑₐᵣₜₕ_ₐᵥₑᵣₐ𝓰ₑ = ₄√( 345,83 Wm⁻² (1 – 0,30 )/ 5,67 × 10⁻⁸ Wm⁻² K⁻⁴) = 255,62° K or -17.53° C
space.com/24854-how-old-…
oceanservice.noaa.gov/facts/why_ocea…

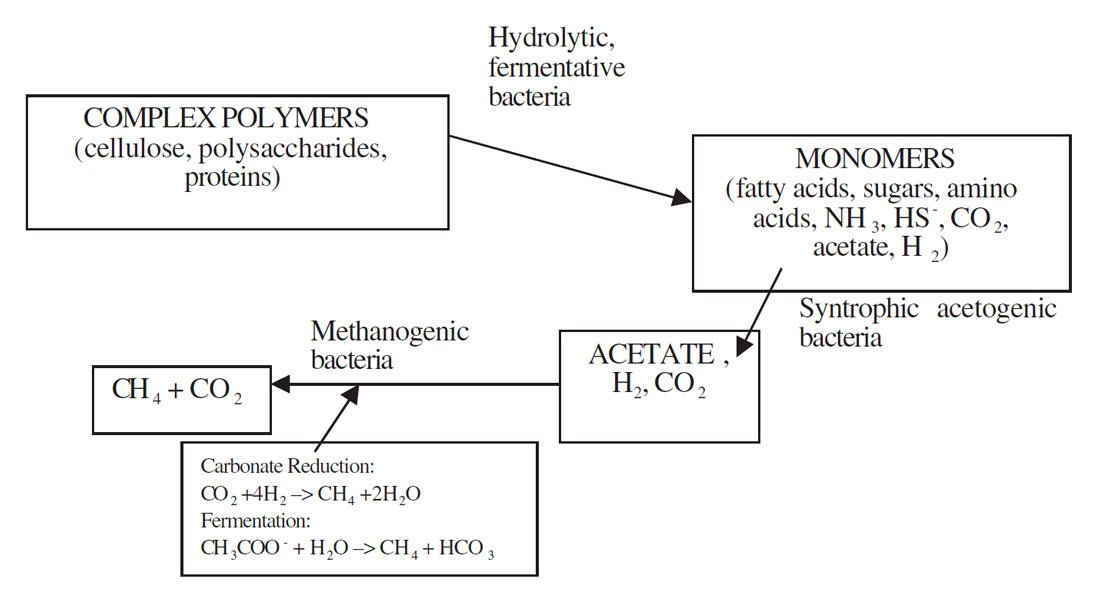
Acetic acid CH₃COOH => CO2 + CH4
csegrecorder.com/articles/view/…
phys.org/news/2017-03-e…
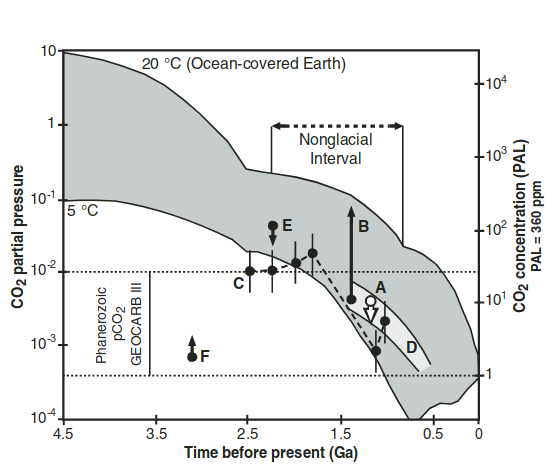
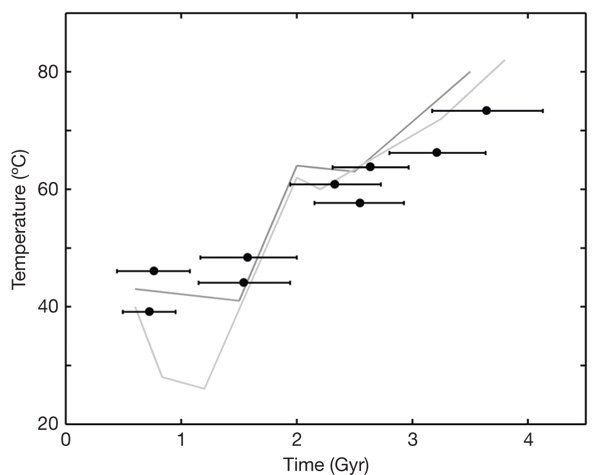
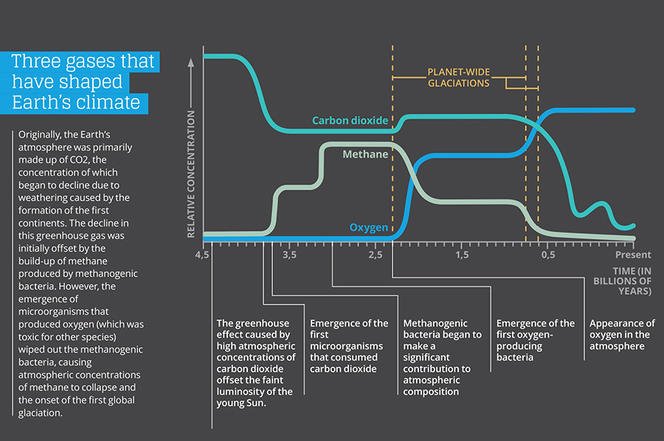
(~353 K to ~ 353 K) from ~ 3.8 billion year ago till the Great oxidation event changed the atmosphere.
researchgate.net/publication/55…
CH₃COOH => CO₂ + CH₄
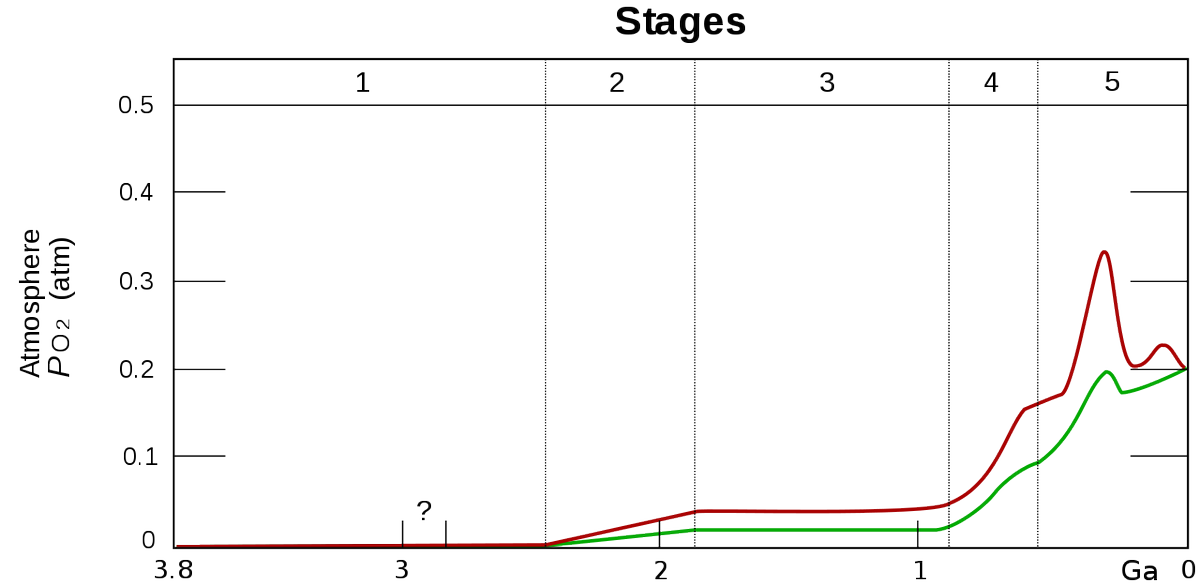
source : en.wikipedia.org/wiki/Great_Oxi…
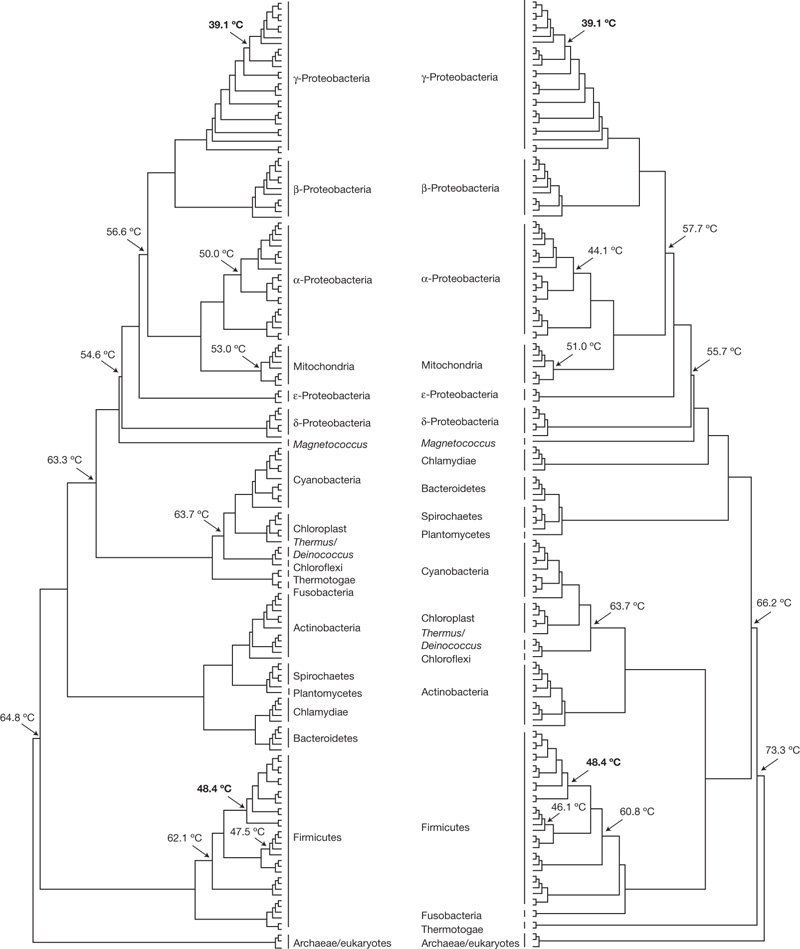
researchgate.net/figure/Cladogr…
"Artist's impression of early Earth (Hadeano)"
sci-news.com/geology/early-…

scitechdaily.com/earth-loses-50…
PV=nRT
Pressure (P),
Volume (V),
the number of substance in gas (n),
Gas constant (R),
Temperature (T)
n = m/M
Molar mass atmosphere (M) = 28,97 g/mol
n = m/M
= 5,1×10²¹ g ÷ 28,97 g/mol
= 1,760441836×10²⁰ mol
V = nRT/P = 4,157289337×10¹⁸ m³
T at 1014 hPa = 14,85° C or 288 K
Nasa Earth Fact Sheet : nssdc.gsfc.nasa.gov/planetary/fact…
source picture :news.cnrs.fr/articles/when-…
giss.nasa.gov/research/featu…

101400 J m⁻³ 4,157289337×10¹⁸ m³/
(353,15 K 8,3144598 J K⁻¹ mol⁻¹ )
= 1,435671099×10²⁰ mol
M = m/n
5,1×10²¹ g ÷ 1,435671099×10²⁰ mol
= 35,52 g mol⁻¹
101400 J m⁻³ 4,157289337×10¹⁸ m³/
(333,15 K 8,3144598 J K⁻¹ mol⁻¹ )
= 1,521858768×10²⁰ mol
M = m/n
5,1×10²¹ g ÷ 1,435671099×10²⁰ mol
= 33,51 g mol⁻¹
Molar mass CH₄ = 16,04 g mol⁻¹
An atmosphere with 70% CO₂ and 30% CH₄
(44,01g mol⁻¹×0,7)+(16,04 g mol⁻¹×0,3)= 35,619 g mol⁻¹
An atmosphere with 60% CO₂ and 40% CH₄
(44,01g mol⁻¹×0,6)+(16,04 g mol⁻¹×0,4)= 32,82 g mol⁻¹
n= PV/TR
101400 J m⁻³ 4,157289337×10¹⁸ m³/
(273,15 K 8,3144598 J K⁻¹ mol⁻¹ )
= 1,856149547×10²⁰
M = m/n
5,1×10²¹ g ÷ 1,856149547×10²⁰ mol
= 27,47 g mol⁻¹
Molar mass CH₄ = 16,04 g mol⁻¹
Molar mass O₂ = 32 g mol⁻¹
An atmosphere with 20% CO₂ and 50% CH₄ and 30% O₂
(44,01g mol⁻¹×0,2)+(16,04 g mol⁻¹×0,5)+ (32 g mol⁻¹×0,3)
= 26,422 g mol⁻¹
sciencedirect.com/science/articl…
Molar mass CH₄ = 16,04 g mol⁻¹
Molar mass O₂ = 32 g mol⁻¹
Molar mass N₂ = 28,01 g mol⁻¹
10% CO₂ and 10% CH₄ and 30% O₂ and 50% N₂
(44,01g mol⁻¹×0,1)+(16,04 g mol⁻¹×0,1)+ (32 g mol⁻¹×0,3)+(28,01 g mol⁻¹×0,5)
= 29,61 g mol⁻¹
CO₂+CH₄ life flourish using CO₂ producing O₂, kills 99% of all life , flourish and transforms atmosphere to
CO₂+CH₄+O₂ and freezes the earth till it tips, life flourish use CO₂ stops CH₄ add O₂, kills 85% of all life.
It's not so bad to add a bit of CO₂.
earthobservatory.nasa.gov/glossary/all
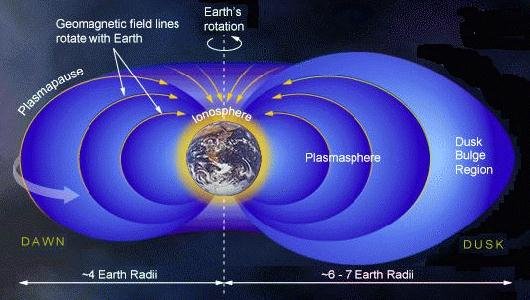
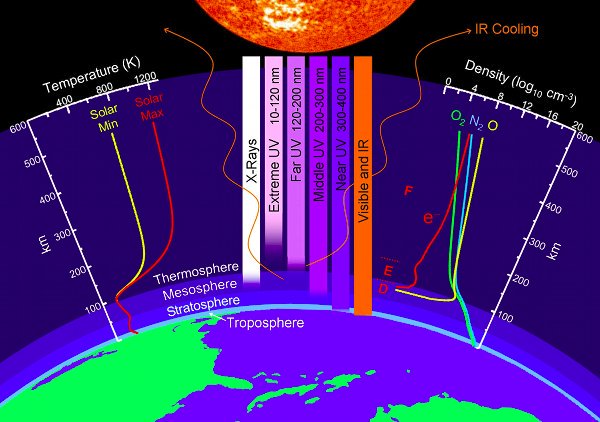
Molar mass He = 4,00 g mol⁻¹
Molar mass CO₂ = 44,01 g mol⁻¹
Molar mass CH₄ = 16,04 g mol⁻¹
Molar mass N₂ = 28,01 g mol⁻¹
Molar mass O = 16 g mol⁻¹
Molar mass O₂ = 32 g mol⁻¹
Molar mass O₃ = 48 g mol⁻¹
universetoday.com/40451/exospher…
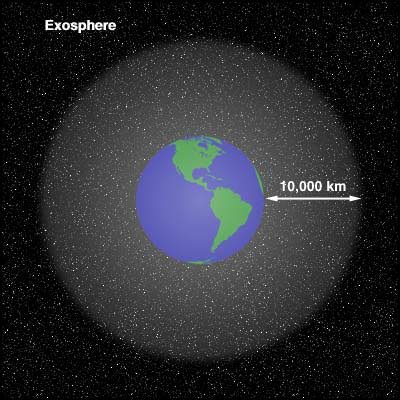
Gas constant (R) 8,3144598 kg m² s⁻² K⁻¹ mol⁻¹
Mesopause coldest place on earth 173 K (T)
Molar mass H, M= 0,00101 kg mol⁻¹
v = √ (3 RT/M)
v =√(3×8,3144598 kg m² s⁻² K⁻¹ mol⁻¹×173 K÷0,001kg mol⁻¹) = 2077,307063532 m s⁻¹
The time before the hydrogen stops and start falling down can be calculated by gravitational acceleration
g = 9,81 m s⁻²
t = v ÷ g
= (2077,31 m s⁻¹) / (9,81 m s⁻²)
= 211,97 s
r = 1/2 gt²
r = 1/2 (9,81 m s⁻²) (211,97 s)²
= 220388,15 m or 220 km
Thermosphere 80km to 600km
v =√(3×8,3144598 kg m² s⁻² K⁻¹ mol⁻¹× 255 K÷0,029 kg mol⁻¹) = 468,33 m s⁻¹
t = v ÷ g
= (468,33 m s⁻¹) / (9,81 m s⁻²)
= 47,74 s
r = 1/2 (9,81 m s⁻²) (47,74 s)² = 11178,88 m the boundary of our atmosphere.
Molar mass atmosphere , M= 0,044 kg mol⁻¹
v =√(3×8,3144598 kg m² s⁻² K⁻¹ mol⁻¹× 737 K÷0,044 kg mol⁻¹) = 468,33 m s⁻¹
t = v ÷ g
= (646,37 m s⁻¹) / (8,87 m s⁻²)
= 72,85 s
r = 1/2 (8,87 m s⁻²) (72,85 s)² = 26035 m or maximum boundary of the atmosphere of Venus

















