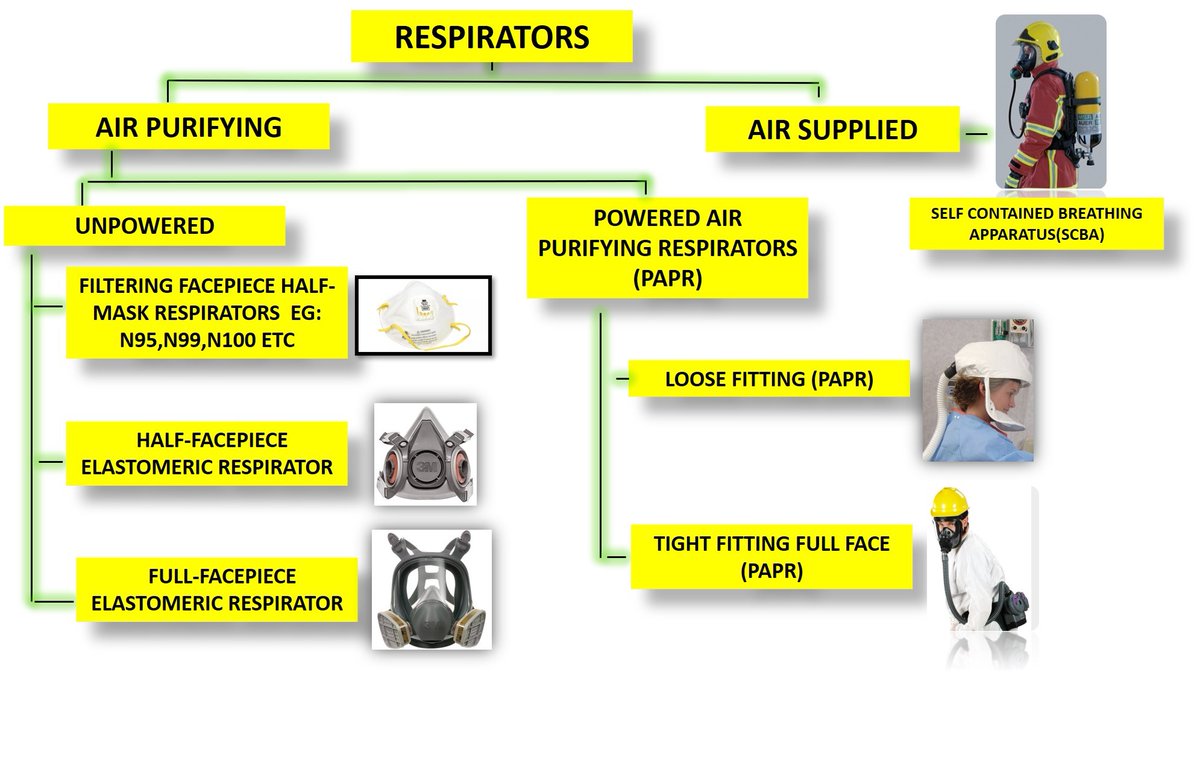
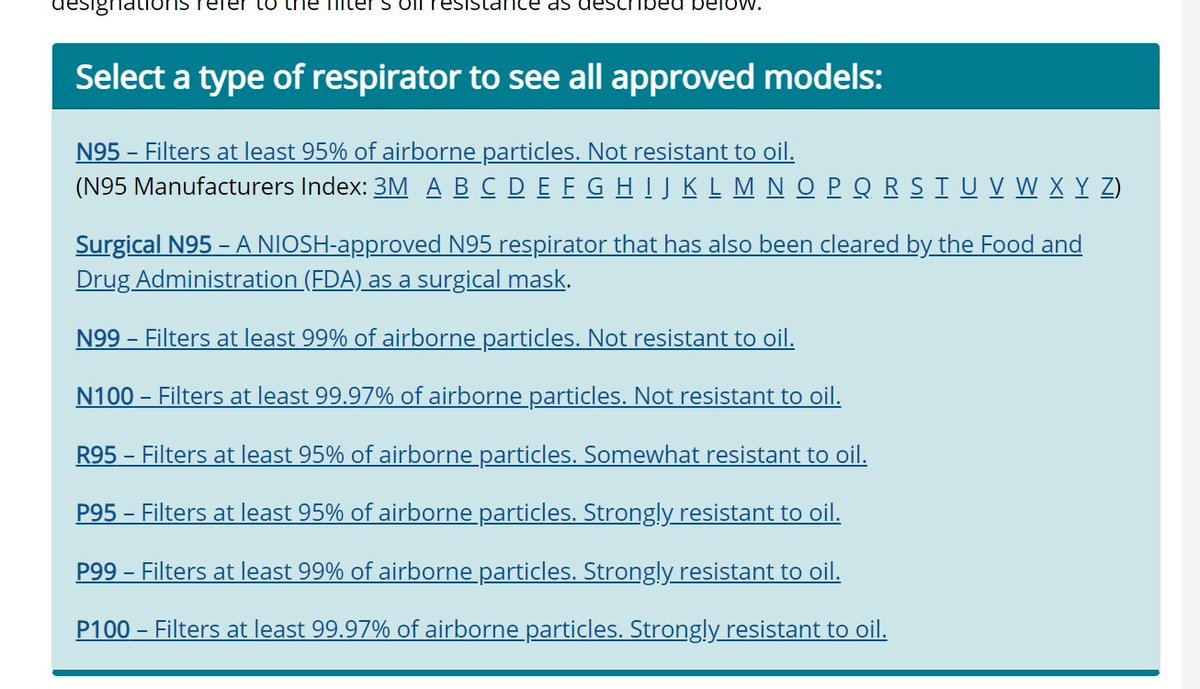
cdc.gov/niosh/npptl/to…
mohfw.gov.in/pdf/Guidelines…
#N95 #PPEkits #PPE #Covid19India
fda.gov/medical-device…
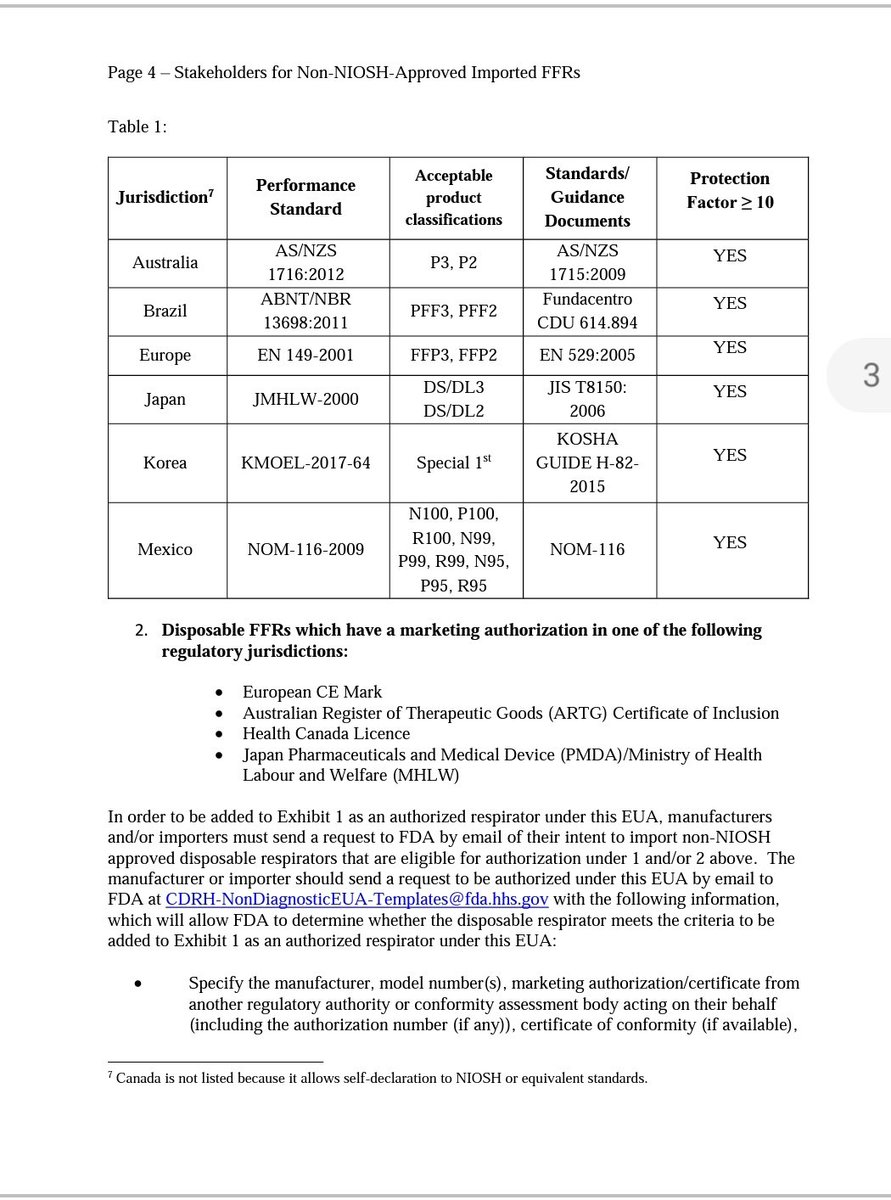
fastcompany.com/90479846/the-u…
fda.gov/media/136403/d…
fda.gov/media/136663/d…
fda.gov/media/136731/d…
beckershospitalreview.com/supply-chain/f…
N95 masks, based on the same principle as STERIS and Battelle. This system claims to decontaminate N95 masks upto 2 times.
businesswire.com/news/home/2020…
fda.gov/media/136976/d…

@MoHFW_INDIA @drharshvardhan

amazon.in/dp/B087TSXLJB/…