Should we use #Remdesivir (RDV) to treat #SARSCoV2? #COVID19 Let’s take a look at the data.
Here’s: #HowIReadThisPaper on the @Lancet trial of RDV for COVID19 in humans
Wang et al: thelancet.com/journals/lance…
(Thread)

First, let’s assess our baseline beliefs about RDV.
Before reading this study, when considering remdesivir as a treatment for patients with severe #COVID19, I think the:
Background: There are currently no effective antivirals for patients with severe COVID19.
RDV, a nucleotide analogue, has broad antiviral activity against many coronaviruses in vitro.
This is the first published clinical trial of RDV for #COVID19 in humans.
Question: Is RDV safe and effective for reducing time to clinical improvement in patients hospitalized with #COVID19?
Date Published: 29 April 2020
Funding: Chinese Academy of Medical Sciences
Study Design: Randomized, double-blind, placebo-controlled superiority trial
Population: 237 adults hospitalized with RT-PCR-confirmed #SARSCoV2 at 10 hospitals in Wuhan, China were included; 158 received RDV, 79 received placebo
Study Period: 2/6/2020 - 3/12/2020
Intervention: RDV 200 mg IV on day 1 + 100 mg IV daily x9 days (10 days total) + usual care
Control: Placebo (10 days total) + usual care
Patients in both groups were allowed to receive other off-label treatments, but not as part of another clinical trial.
Study Procedures: Patients were randomized 2:1 (RDV:placebo) via permuted blocks and stratified by level of O2 support at enrollment.
RNs assessed patients daily and recorded clinical data.
Swabs for PCR were obtained on days 1,3,5,7,10,14,21,28 until discharge or death.
Inclusion Criteria: RT-PCR positive for SARSCoV2 + infiltrates on chest imaging + SpO2 =<94% on room air or P:F ratio <300 mm Hg + <12 days from symptom onset
Exclusion Criteria: cirrhosis, ALT or AST >5x ULN, GFR <30 (or on HD), pregnant or breastfeeding women
Protocol available? Yes: clinicaltrials.gov/ct2/show/NCT04… and
researchsquare.com/article/rs-146…
Primary outcome: Time to clinical improvement from randomization to day 28 (>=2 points ↓ on ordinal scale below, or live discharge)
Secondary outcomes: Mortality, viral load, adverse events
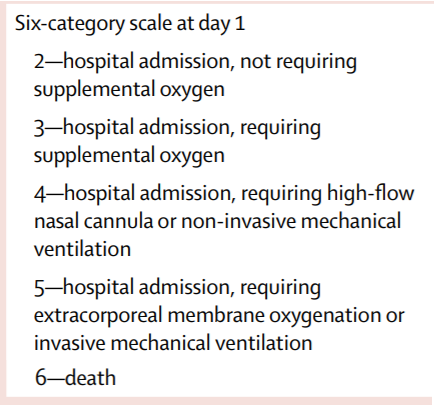
Primary analysis: Intention-to-treat (ITT) survival analysis
Sample size justification: Sample size of 453 = 80% power (w/ 1-sided alpha 0.025) to detect =>6 day reduction in time to clinical improvement, assuming a median of 21 days to clinical improvement in placebo group
Author’s conclusions: RDV was not associated with reductions in time to clinical improvement, viral shedding, or mortality in hospitalized patients with #COVID19; however, the study was underpowered for the primary outcome. Serious adverse events due to RDV were rare.
Let’s reframe the original question, this time focusing on the benefits of RDV:
After reading this study, I think the authors’ estimate of the benefits of RDV in patients with severe #COVID19 is likely to be:
My appraisal: This was a negative study.
Does this mean that RDV truly has no effect on time to clinical improvement, or could it have an effect that we just couldn’t find?
Let’s look for sources of chance and bias that could make a false negative more likely.
Regarding chance: because the outbreak came under control in China, enrollment slowed, and the study was stopped after enrolling only about half the planned sample size.
This left the study underpowered for the primary outcome, which could lead to a false negative result.
A smaller-than-planned N in a superiority RCT can compromise both accuracy and precision of the estimate of the treatment effect.
This study was powered to find a HR for improvement of 1.4, assuming a median time to clinical improvement of 21 days in the placebo group.
Enrolling 237 of 453 planned patients made it unlikely that any effect smaller than what was assumed in the power calculations could reach statistical significance.
A larger (more obvious) effect size would need to exist in order for fewer observations to reveal it.
These authors recently published a well-designed RCT on lopinavir-ritonavir in @NEJM that was also underpowered, but for a different reason (the control group improved faster than expected). This did not happen in this study.
Regarding bias: Due to small size, randomization was imperfect, and imbalances in baseline characteristics suggest patients in the RDV group were sicker & farther along:
↑ comorbidities
↑ tachypnea
↑ lower respiratory tract viral load
↑ % of patients with Sx for >10 days
Patients being sicker in the RDV group could attenuate any real benefits of RDV (if any exist) and make a false negative result more likely.
For these reasons, it is not surprising that reductions in mortality or viral shedding were also not shown.
Finally, there was no difference in viral load reduction between groups.
This could reflect RDV having no effect on viral load, or RDV being given too late in the disease.
Note that detection of viral RNA is not a reliable surrogate for the presence of infectious virus.
Finally, let’s revisit the question in tweet 18:
After critically appraising this study, I think the authors’ estimate of the benefits of RDV in patients with severe #COVID19 is likely to be:
Bottom line: RDV was not associated with reduced time to clinical improvement in patients hospitalized with #COVID19. Lack of power due to early stopping and baseline imbalances suggesting the RDV group was sicker both raise the likelihood of a false negative result.
(End)
After critically appraising this study, I think the authors’ estimate of the benefits of RDV in patients with severe #COVID19 is likely to be: