Should we use #remdesivir (RDV) to treat #COVID19 #SARSCoV2? Let’s take a look at the data.
Here’s: #HowIReadThisPaper on the @NEJM case series of remdesivir for COVID19:
Grein et al: nejm.org/doi/full/10.10…
(Thread)

Background: RDV is an ATP analogue that inhibits many viral RNA polymerases in vitro, including MERS-CoV and SARS-CoV1+2. However, in an RCT of 4 drugs for ebola, RDV was ineffective:
nejm.org/doi/full/10.10…
It does not currently have an FDA-approved indication for use.
Question: What were the outcomes among patients hospitalized with #COVID19 who received RDV on a compassionate use basis?
Date Published: 10 April 2020
Funding: Gilead Sciences
Study design: Case series
Population: 61 patients hospitalized in several countries with severe #COVID19 for whom compassionate use of RDV was approved by Gilead
(more on compassionate use, or expanded access: fda.gov/news-events/pu…)
Study period: 1/25/2020 - 3/30/2020
Intervention: RDV 200 mg IV on day 1 + 100 mg IV daily x9 days (10 days total) in addition to usual care, which varied at each hospital, but at a minimum included non-invasive or invasive oxygen support
Control: none
Study procedures: Treating clinicians submitted an application for RDV to Gilead. Approved patients received a 10-day course, plus usual care as determined by treating clinicians. Clinical and laboratory data were reported daily through day 10, and sporadically thereafter.
Inclusion criteria: Hospitalized patients with RT-PCR confirmed #SARSCoV2 + SpO2 =< 94% on room air or need for supplemental O2
Exclusion criteria: Renal impairment (CrCl <30 mL/min), active hepatitis (AST or AST >5x ULN), use of any other investigational drug for COVID19
Protocol available? Yes (nejm.org/doi/suppl/10.1…)
Primary outcome: None specified
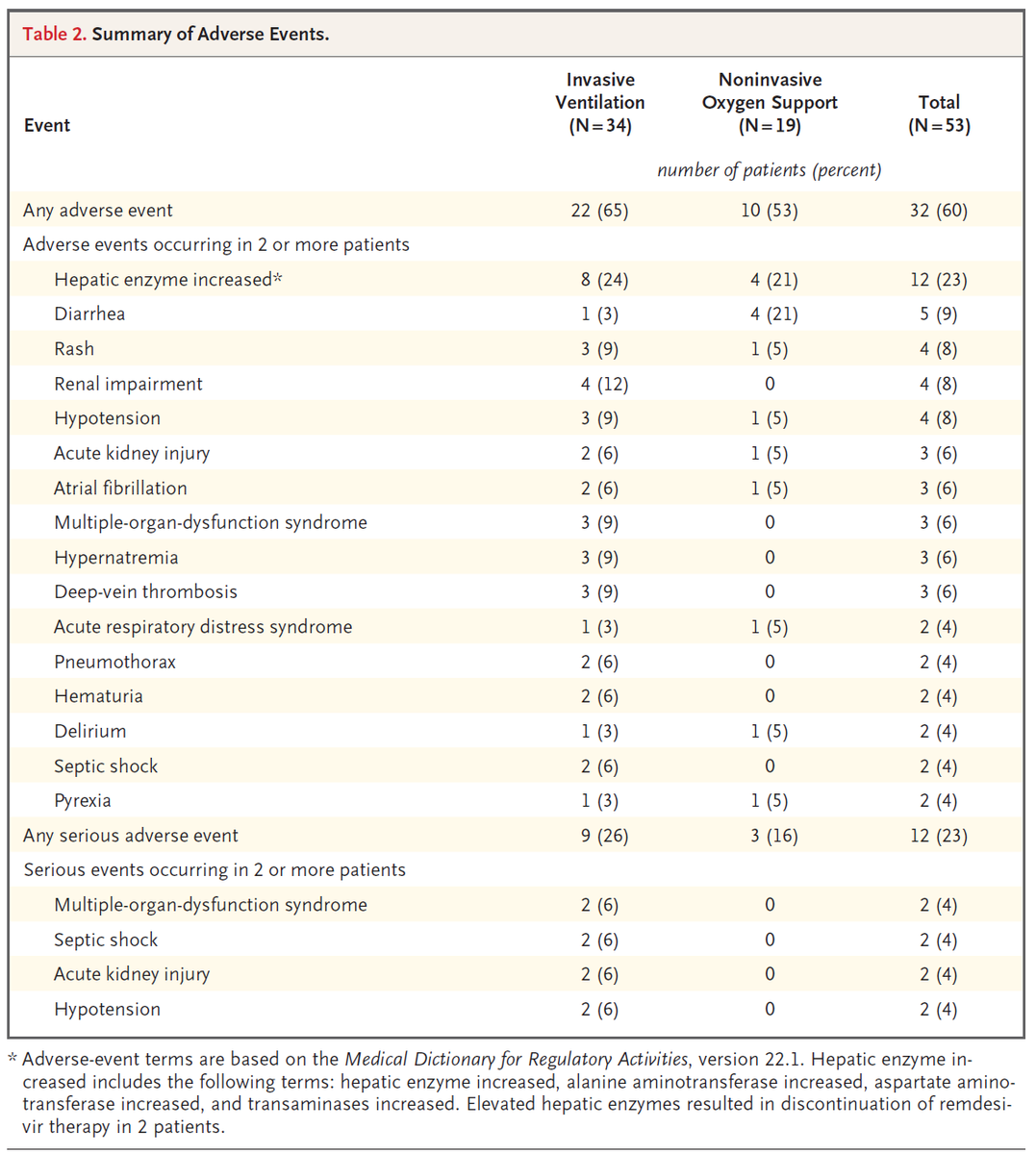
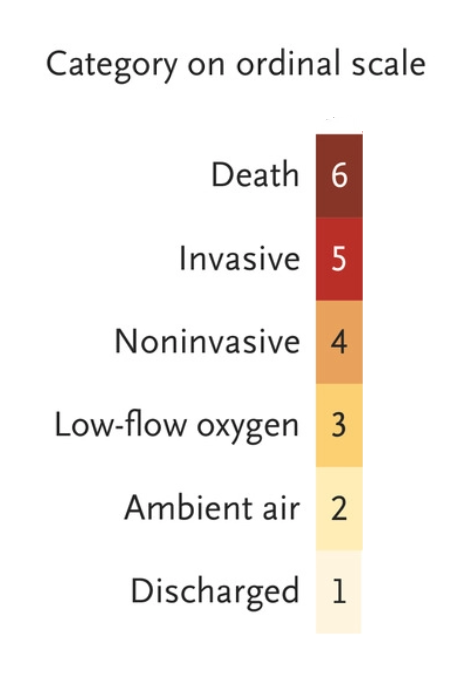
Other outcomes reported: Cumulative incidence of clinical improvement (↓ =>2 levels on scale below or live discharge); change in oxygen support, adverse events, mortality, and lab data
Primary analysis: Descriptive only
Sample size justification: Not provided
Duration of follow-up: 28 days
Loss to follow-up: 8/61 patients (7 patients contributed no post-treatment data; 1 patient received an incorrect dose of RDV).
Author’s conclusions: Despite the lack of a control group, comparing the observed mortality rate of 13% in this severely ill patient group with that seen in other published reports suggests RDV may have benefits in patients with severe COVID19.
My appraisal: The authors note small size, short follow-up, missing data, and lack of a control group as the main limitations of this study. Accepting those problems, are there other sources of bias to threaten the suggestion that RDV is promising?
Yes. Let’s take a look.
It is tempting to think that descriptive studies are less vulnerable to bias than analytical studies, but let’s see why this is not so.
Two important questions to ask here are:
1. How did patients get into the study?
2. How did outcomes come out of the study?
The first question is all about looking for selection bias. For a patient to be in this study:
Clinicians had to apply for RDV (n =?)
Gilead had to approve RDV (n =61)
Patients had to complete the RDV protocol (n =53)
Each step introduces the potential for selection bias.
In addition, patients who receive an expanded access medication are likely to differ from those who do not, and clinicians who submit an application are likely to differ from those who do not.
This makes it difficult to know who to generalize the results of this study to.
Further, the median time from Sx onset to receipt of RDV was 12 days. This means patients had to survive the first 12 days of COVID19 to be eligible for this study.
An early series from Wuhan reported a median time from Sx onset to ARDS of 8 days:
jamanetwork.com/journals/jama/…
Once patients made it into this study, the lack of a standardized definition of “usual care” is another potential source of bias.
Could interventions other than RDV explain the 13% mortality rate, apparently lower than in other series? We don’t know.
For comparison, the authors cite a mortality rate of 66% among intubated patients in another series:
jamanetwork.com/journals/jamai…
In a subsequent larger series (n=1,591) mortality among ICU patients was 26% (of whom 88% were intubated):
jamanetwork.com/journals/jama/…
The 2nd Q in tweet 18 is all about ascertainment bias.
Fig 2 raises concern that patients who were sicker at baseline were less likely to be followed until clinical improvement, discharge, or death.
Of the 8 patients with none of those outcomes reported, all were intubated.
The most likely situation is that they were still hospitalized at the end of the study period, but because they did not contribute complete person-time data, we don’t know what happened to them. Because they were sicker, this creates ascertainment bias.
Regarding adverse events, it is difficult to know whether these are due to COVID19 or RDV. The most informative adverse events are those that prompted discontinuation of RDV, which occurred in 8% of patients.
Bottom line: Without a control group, it is impossible to tell whether the observed outcomes in this selected population are due to RDV. Selection bias limits both comparison to other cohorts and the generalizability of these results. Well-designed RCTs are needed.
(End)