Should we use #lopinavir-ritonavir (LPV/r) to treat #COVID19 #SARSCoV2? Let’s take a look at the data.
Here’s: #HowIReadThisPaper on the @NEJM trial of LPV/r for COVID19 in humans: LOTUS China
Cao et al: nejm.org/doi/full/10.10…
(Thread)
Background: Lopinavir, an HIV protease inhibitor, is active in vitro against SARS-CoV and in animal models of MERS-CoV. Ritonavir inhibits CYP3A and increases serum half-life of lopinavir. A 2004 uncontrolled study of LPV/r + ribavirin suggested a benefit in SARS in humans.
Question: Is LPV/r safe and efficacious for shortening time to clinical improvement in patients hospitalized with #COVID19?
Date Published: 18 March 2020
Funding: Major Projects of National Science and Technology on New Drug Creation and Development
Study Design: Randomized, open-label (unblinded) superiority trial
Population: 199 adults hospitalized at a single center with RT-PCR confirmed #SARS-CoV-2 were included; 99 were assigned LPV/r, 100 were assigned usual care.
Study Period: 1/18/2020 - 2/3/3030
Intervention: LPV/r 400-100 mg BID for 14 days + usual care ( = any combination of supplemental O2, invasive or noninvasive ventilation, antibiotics, vasopressors, renal replacement therapy, or ECMO)
Control: usual care alone
Study Procedures: Patients were randomized 1:1 via permuted blocks and stratified by level of O2 support at enrollment. RNs assessed patients daily and clinical data were recorded. OP swabs were obtained on days 1,5,10,14,21,28 until discharge or death.
Inclusion Criteria: RT-PCR from a respiratory sample positive for SARS-CoV-2 + infiltrates on chest imaging + SpO2 =<94% on room air or P:F ratio <300 mm Hg.
Exclusion Criteria: Allergy to LPV/r, cirrhosis + ALT or AST >5x ULN, known HIV, pregnant or breastfeeding women
Protocol available? Yes: chictr.org.cn/showprojen.asp…
Primary outcome: Time to clinical improvement from randomization (=>2 points on the ordinal scale below)
Secondary outcomes: Included mortality by day 28, viral RNA detection over time, adverse events
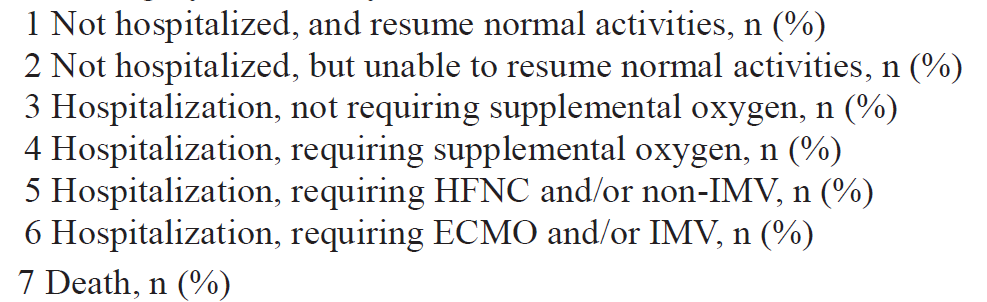
Primary analysis: Intention-to-treat (ITT) survival analysis
Power calculations: Sample size of 160 = 80% power (w/ 2-sided alpha 0.05) to detect =>8 day reduction in median time to improvement, assuming a median of 20 days to clinical improvement in the control group.
Duration of follow-up: 28 days
Loss to follow-up: 0/99 LPV/r patients (94/99 actually received LPV/r); 0/100 usual care patients (100/100 actually received usual care)
Table 2:
LPV/r vs usual care:
Median days from Sx onset to enrollment: 13 vs 13
Mean log viral load at enrollment: 4.4+/-2.0 vs 3.7+/-2.1
Tx with:
Steroids: 32% vs 35%
Abx: 95% vs 95%
Pressors: 17% vs 27%
NIV: 10% vs 19%
Author’s conclusions: Addition of LPV/r to usual care did not shorten the time to clinical improvement in patients hospitalized with #COVID19 compared with usual care.
My appraisal:
This was a negative study. Could chance or bias explain that result? Put another way, are we confident the benefit of LPV/r we sought does not exist, or could it exist and we just didn't find it?
The latter could happen if the study was underpowered for the primary outcome - initially, it was, but the authors tried to address this. They increased enrollment by about 25% (from 160 to 199) for this reason. So, a type II error seems less likely.
Why did that happen? One possibility is that the usual care group improved faster than expected (16 days observed vs 20 days expected). This left less time for the LPV/r group to improve faster and demonstrate benefit over usual care.
How much faster would the LPV/r group have needed to improve to show benefit? The study was powered to find a reduction in time to clinical improvement of 8 days or more. This is a relatively large effect size, and a smaller reduction might still be clinically meaningful.
Overall, baseline characteristics were well-balanced. Permuted block randomization was used appropriately given the small size, and stratification by level of respiratory failure at enrollment ensured balance of this known covariate between groups.
Of note, these were moderately sick patients relatively late into their disease course. The overall mortality was 22%, which is higher than the 10-15% reported in other early case series of hospitalized patients:
ncbi.nlm.nih.gov/pubmed/3200714…
ncbi.nlm.nih.gov/pubmed/3198626…
Despite that, almost nobody was intubated (1%), so it is hard to draw conclusions about patients with very severe respiratory failure requiring intubation. Similarly, whether LPV/r could have benefit in patients earlier in their disease course is not answered by this trial.
Only 7 patients were right-censored at day 28 (meaning they did not die or experience the primary outcome before this) suggesting that 28 days was an adequate duration of follow-up to capture most events.
The results of the primary, ITT analysis are the strongest. This is because they bias toward the null and are less vulnerable to type I error (testing multiple secondary outcomes increases this risk overall) and type II error (a study being underpowered increases this risk).
For this reason, all secondary outcomes and post-hoc analyses should be considered hypothesis-generating only. The suggestion of a 1-day reduction in time to improvement in a post-hoc per-protocol analysis is intriguing, but less useful than the negative primary outcome.
Bottom Line: Added to usual care, LPV/r did not result in a large (=>8 day) reduction in time to clinical improvement in patients hospitalized with #COVID19. Further study is needed to assess benefit of LPV/r in other settings (e.g. in prevention and mild disease).
(End)











