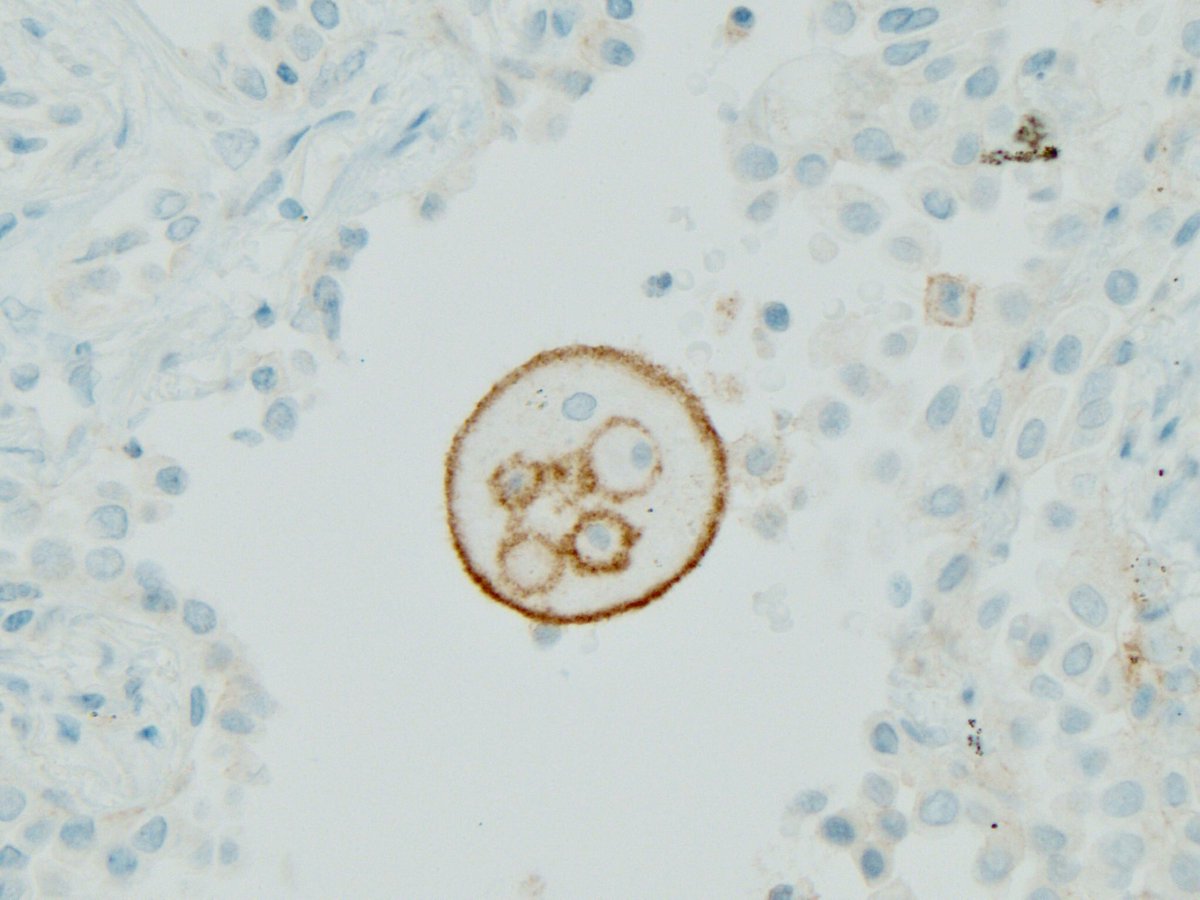
It’s correct to say we should use all available clinical, radiologic and pathologic information, but that’s too vague to be helpful in a practical sense.
Here I offer some tips. #tweetorial
IMHO it’s a mistake to approach a carcinoma in the lung as carcinoma of unknown primary
💥Clinicians: give your pathologists this information! If you don’t, this is substandard, dangerous patient care.
Also, pathologists don’t always grasp the clinical question.
This is a recipe for confusion.
Not: “is this a metastasis from an occult primary?”
And if the sample is a biopsy you’re wasting precious tissue.
If you want to do ihc just to confirm it’s not lung that’s ok.
“But I never get any clinical history!!” some will say. Well then, your clinicians are putting patients in danger.
ALWAYS consider a met in this situation, even if the mass is large and solitary. #IHCpath can be very helpful (know your pitfalls)
Too bad. Late mets to lung do happen. As long as 33 years later. Especially with RCC and endometrial carcinoma.
pubmed.ncbi.nlm.nih.gov/28248821/
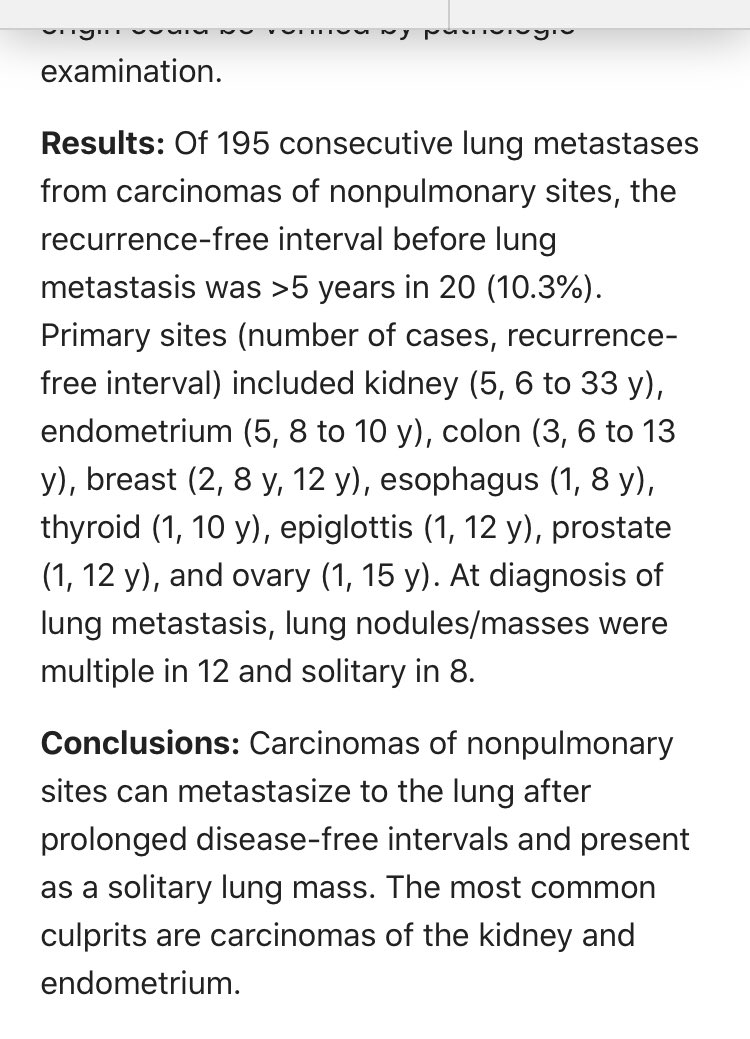
Use your judgement. Some of these cases will require #ihcpath and some won’t. But always at least consider a met.
Next tweets will address situations without a history of carcinoma...
If you get a lung biopsy with carcinoma in this uncommon situation by all means use #ihcpath
You could but your yield for finding mets will be near zero
🔥 solitary lung mass
💥 no history of prior carcinoma
I don’t do any #ihcpath here but many people do. What do they find? TTF-1 pos in 80% cases, thus answering a question no one asked
How often do they find mets? Zero.
How often do PET scans find an occult primary in the scenario outlined in tweet 16?
Zero.
I have asked in public for such a paper for years.
How many are there?
How many such cases have I seen?
One exchange with my friend @BinXu16 is still with me to this day.
These are usually residents, or bright folks with a strong research or basic science background. They believe in objective facts, not fuzzy clinical data or fuzzy supreme H&E.
TTF-1 positive = lung origin
TTF-1 negative = not lung origin
The problem is, neither of these statements are true.
TTF-1 stains thyroid, GYN, even rare colon and breast carcinomas.
Colon by @BinXu16
pubmed.ncbi.nlm.nih.gov/20042854/
Cholangiocarcinoma
pubmed.ncbi.nlm.nih.gov/24418856/
Breast (Juan Rosai)
pubmed.ncbi.nlm.nih.gov/21677546/
Prostate, salivary, colon
pubmed.ncbi.nlm.nih.gov/19887917/
You didn’t know TTF-1 was this “promiscuous”, did you? 😳😢
Sorry, napsin A also stains thyroid, RCC and GYN carcinomas especially clear cell carcinomas.
The polyclonal antibody will even stain GI cancers!
This is where PAX-8 is helpful.
An adenocarcinoma pos for TTF-1 and napsin A but neg for PAX-8 is likely to be from the lung.
Which brings us back to the need for clinical and imaging info. You gotta have it.
The frequency of TTF-1 and napsin A in primary lung adenocarcinoma is approximately 80%.
What of the other 20%? Many folks write a comment: “cannot exclude an upper GI or pancreatic primary”
Clinicians:
How many times have I seen an occult upper GI or pancreatic primary found this way in the context of a solitary lung mass?
Then what good is the TTF-1 stain in this situation? Positive doesn’t confirm. Negative doesn’t exclude.
Nope.
The list of #ihcpath that can be expressed in lung cancer:
🔥 GATA-3
🔥 CDX-2
🔥 ER
🔥 CA19-9
🔥 CK19
🔥 SALL4
The only exception is strong and diffuse PAX-8
pubmed.ncbi.nlm.nih.gov/28777151/
Nope. You will create even bigger problems because this antibody will be super promiscuous. See SP141
Should CDX-2 staining prompt a colonoscopy?
Your clinician will say:
The issue is subtyping, not site of origin.
journals.lww.com/ajsp/fulltext/…
We must use it as @natasharekhtman says. If a tumor has papillary structures and psammoma bodies, you gotta think thyroid. But lung cancer can have psammoma bodies & papillary structures too
But then you must return to clinical/imaging. No renal mass, no RCC.
You will never be able to sell a solitary lung mass as a met from endometrium without an endometrial mass or history.
One man’s “typical lung cancer” is another man’s “enteric looking”. That’s the problem with morphology.
It’s a good debate!
For now, I’ll leave you with the stuff of #ihcpath nightmares. A GYN carcinoma that stains with TTF-1, GATA-3 and calretinin! Sweet dreams!