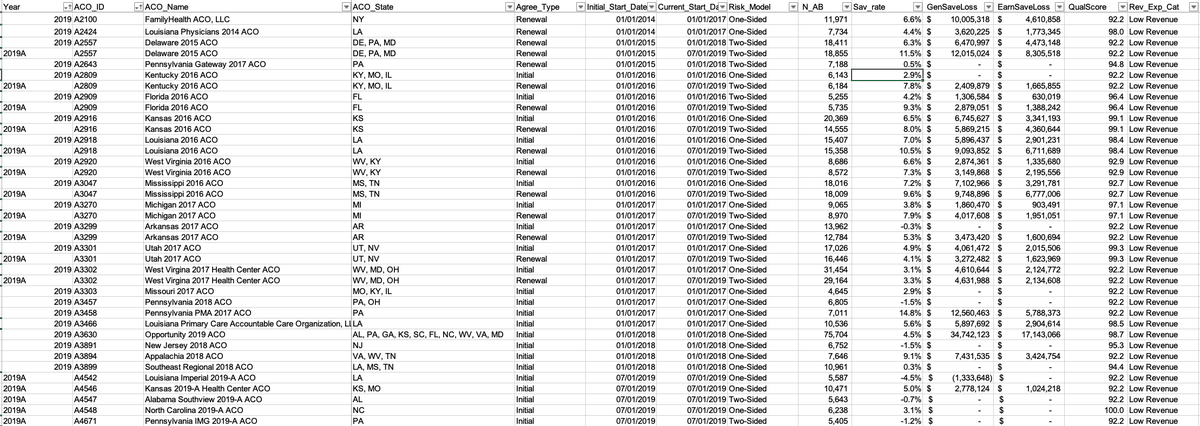
1/ Policy makers: Wait, Isn’t quality of care better at large expensive health systems c/w independent practices?
Previous Research: Ummm no. But we can keep looking
@AHRQNews What if we look at high needs patients?
@RANDCorporation It’s ... worse?
onlinelibrary.wiley.com/doi/abs/10.111…
Previous Research: Ummm no. But we can keep looking
@AHRQNews What if we look at high needs patients?
@RANDCorporation It’s ... worse?
onlinelibrary.wiley.com/doi/abs/10.111…
2/ To test hypothesis that health systems provide better care to patients w high needs, diff in quality b/w system‐affiliated & nonaffiliated physicians
ED visits were significantly *different* in system‐affiliated (117.5 per 100) & nonaffiliated POs (106.8 per 100, P < .0001).
ED visits were significantly *different* in system‐affiliated (117.5 per 100) & nonaffiliated POs (106.8 per 100, P < .0001).
3/ I love how delicately the RAND researchers approach this in their conclusion: “Health systems may not confer hypothesized quality advantages to patients with high needs.”
(Then why do they get paid so much more?)
(Then why do they get paid so much more?)
4/ The usual response to these sort of findings from health systems is that they care for sicker patients.
So this paper only looked at sick patients, and then did every statistical manipulation known to humanity to look for and adjust for differences in the patients
Same diff
So this paper only looked at sick patients, and then did every statistical manipulation known to humanity to look for and adjust for differences in the patients
Same diff

• • •
Missing some Tweet in this thread? You can try to
force a refresh