#breastradpath with @DrJordanaP
For #BreastCancerAwarenessMonth month we want to review the process from imaging to diagnosis of breast cancer. 1/
For #BreastCancerAwarenessMonth month we want to review the process from imaging to diagnosis of breast cancer. 1/

It starts when a patient comes in for a screening or diagnostic mammogram. Here is @DrJordanaP reviewing #mammograms in the reading room. 2/ 

Please review screening modalities in this amazing tweetorial from last week from @DrJordanaP! 3/
https://twitter.com/DrJordanaP/status/1313207983405510657?s=20
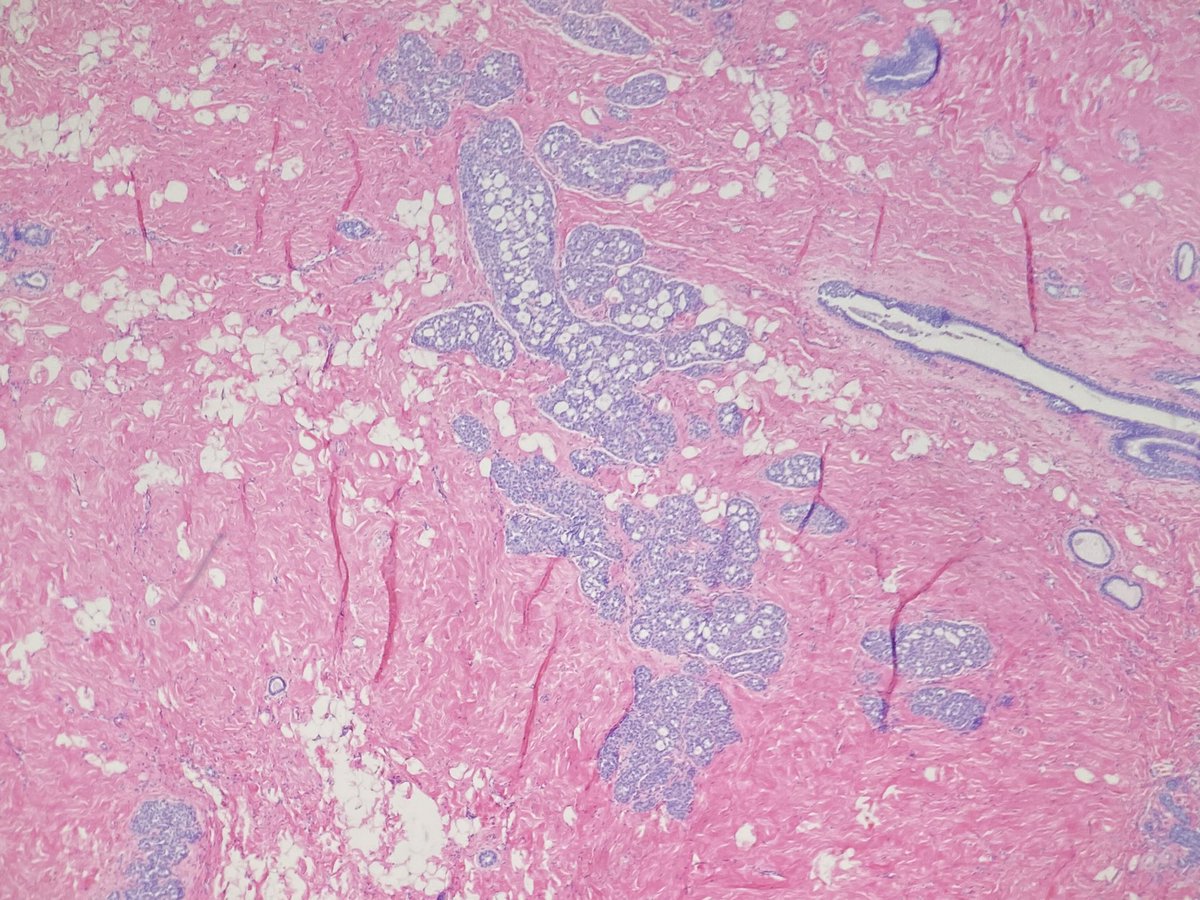
If an area of concern is id'd on imaging (stay tuned for a tweetorial on BI-RADS) that requires further evaluation by sampling, then the patient returns for a bx. At our institution most bxs are core needle bxs (rarely are FNAs performed of primary breast lesions). 4/
The method of biopsy (MG-guided, US-guided, or MRI-guided) and biopsy device depends on the target lesion and how it is best seen. 5/
The patient is prepared and positioned for a biopsy. Here is an example of the tray used for an US-guided core needle biopsy. 6/ 

The biopsy device is used to sample the target lesion. 7/
A metal clip is placed in the mass to indicate the area that was just biopsied. Clips have all different shapes. This example shows a breast cancer ribbon shaped clip! 8/ 

The cores of tissue are placed immediately into formalin. For MG-guided biopsies of calcifications, the specimen is divided in two: one with calcifications and the other without calcifications. 9/ 

For the ASCO/CAP guidelines for evaluation of ER, PR, and HER2, the tissue should be placed in formalin within an hour and remain in formalin for 6-72 hours. 10/
Guidelines were recently updated:
ER and PR : bit.ly/36Xpo3s
HER2: bit.ly/36XLqTV
Guidelines were recently updated:
ER and PR : bit.ly/36Xpo3s
HER2: bit.ly/36XLqTV
The cores are sent to path where they are grossed by our amazing techs. Grossing means the cores placed into tissue cassettes for processing (pic one with arrows). Ours are color coded (pink for breast). Then more formalin (remember, need at least 6 hours in formalin!). 11/ 



Next step is the processor. The cassettes with the tissue go through a series of steps on the processor overnight (there are many steps, but essentially it is to fix the tissue and dehydrate it). Here is processor #2! 12/ 

Embedding is next. 1. A histotech places a little clear liquid paraffin wax into metal well (which acts as a mold). 2. Add tissue. 3. Cassette (this one is grey) placed on top (these are labeled with patient info). Here is gif #1! 13/
4. More liquid paraffin wax is added. 5. Then the block is cooled into a solid wax block with tissue in it. And gif #2! 14/
The blocks with the tissue (tissue blocks) are cut by a histotech into thin ribbons of tissue and paraffin. 15/
The ribbons of tissue float in a water bath where the tissue profiles are separated and picked up by the slides that are labeled with the patient identifiers (this slide is blank as example for this tweetorial). 16/
Here are pics of the tissue after being transferred onto the slides. They are then placed in a small oven to bake the tissue onto the slides and remove that paraffin (it melts away). 17/ 



The slides are then sent through a series of steps to stain the tissue with hematoxylin (the blue stain) and eosin (the pink stain). 18/ 

For microscopic review (next step), we are provided 3 "levels" (tissue slides) for each block. 19/
Info about levels from teaching points in this post:


Info about levels from teaching points in this post:
https://twitter.com/LizaMQuintana/status/1312107067944042496?s=20

The slides make their way to me, the pathologist, who looks at them using my microscope. I review the clinical history and the imaging information when reviewing the slides. Then I make a diagnosis. 20/ 

If there are questions about concordance or about next steps, then the imaging and pathology are reviewed at our weekly Rad Path Correlation Conference. We have been meeting virtually over the past many months. 21/ 
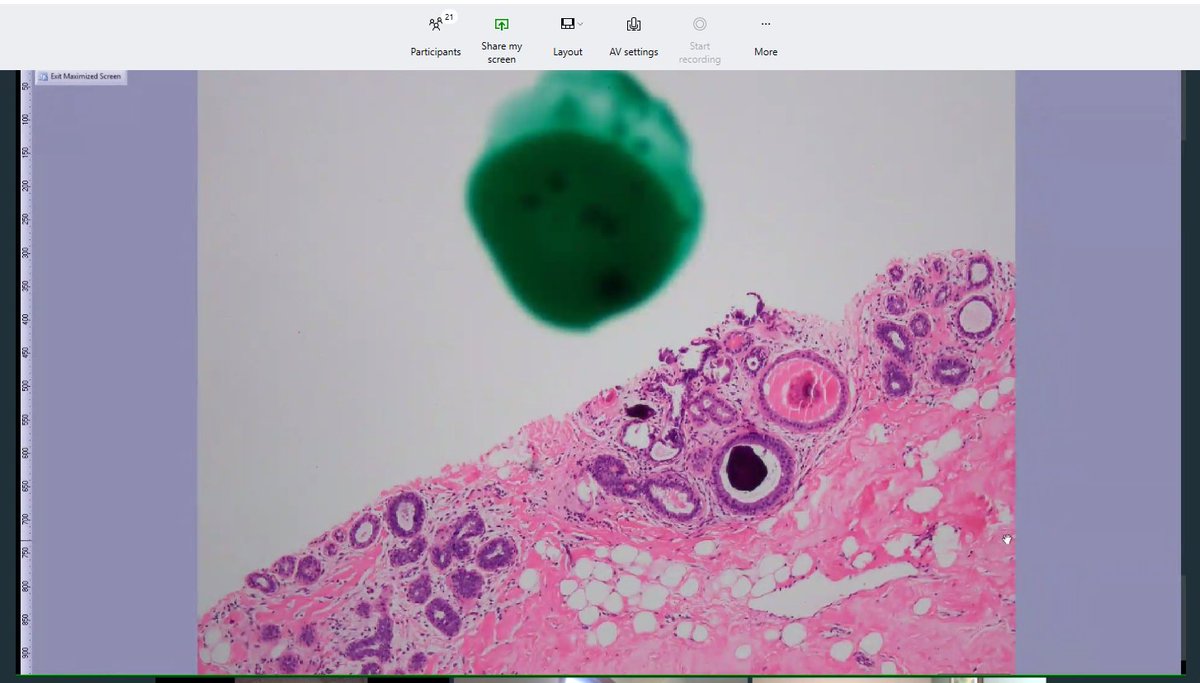
If there is a question of concordance (either when I review the case initially or after Rad Path Conference) then we can go back to get more levels. This means, more tissue ribbons are cut from the tissue block of interest, and more slides are created and stained. 22/
If the specimen has carcinoma, then specific biomarker studies are performed to help guide treatment. (Tweetorial on biomarkers to come!) 23/
Thank you all for following along! This was a long one! If you have any requests of what you would like to see either as a case or a tweetorial let us know! 24/
• • •
Missing some Tweet in this thread? You can try to
force a refresh