Here's plot of how mutating RBD sites affects average serum binding (y-axis) vs frequency of mutations (x-axis). E484K in S African lineage most worrying. But others affect some serum to various degrees & no such thing as "average" human when it comes to serum specificity (13/n) 
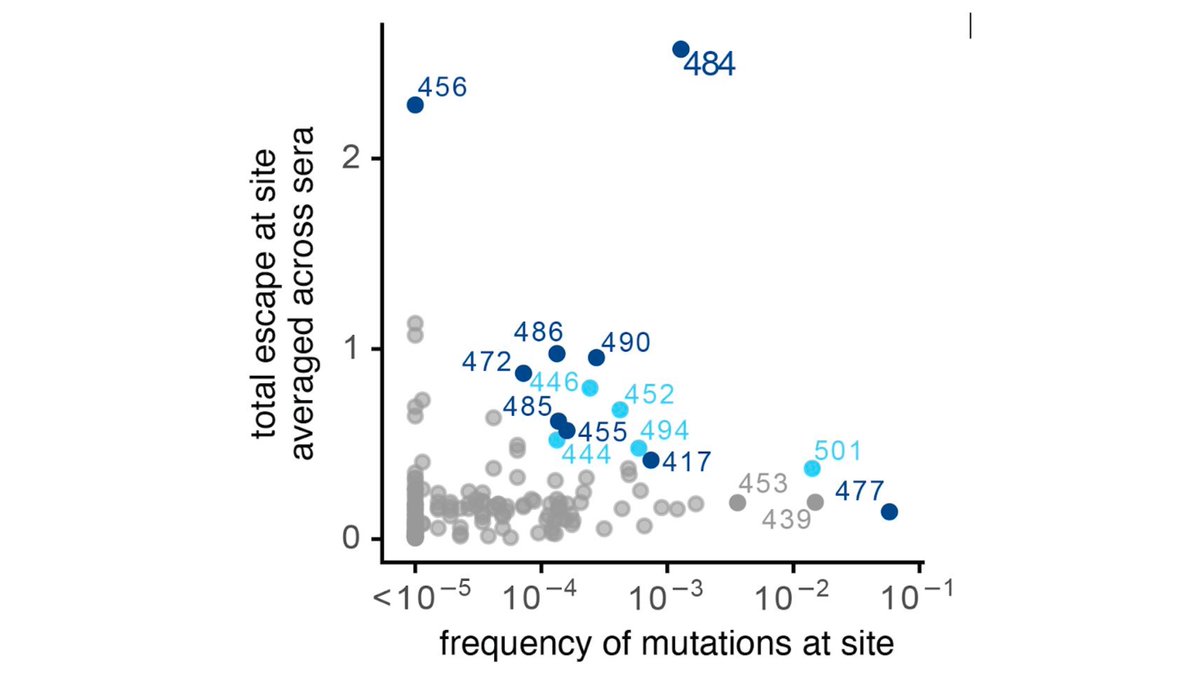
Importantly, we only looked at RBD muts, since majority of neut activity of most sera from RBD antibodies (2nd tweet of thread). But NTD muts also important; see @10queues @mccarthy_kr @GuptaR_lab @e_andreano @McLellan_Lab: biorxiv.org/content/10.110…, medrxiv.org/content/10.110… (14/n)
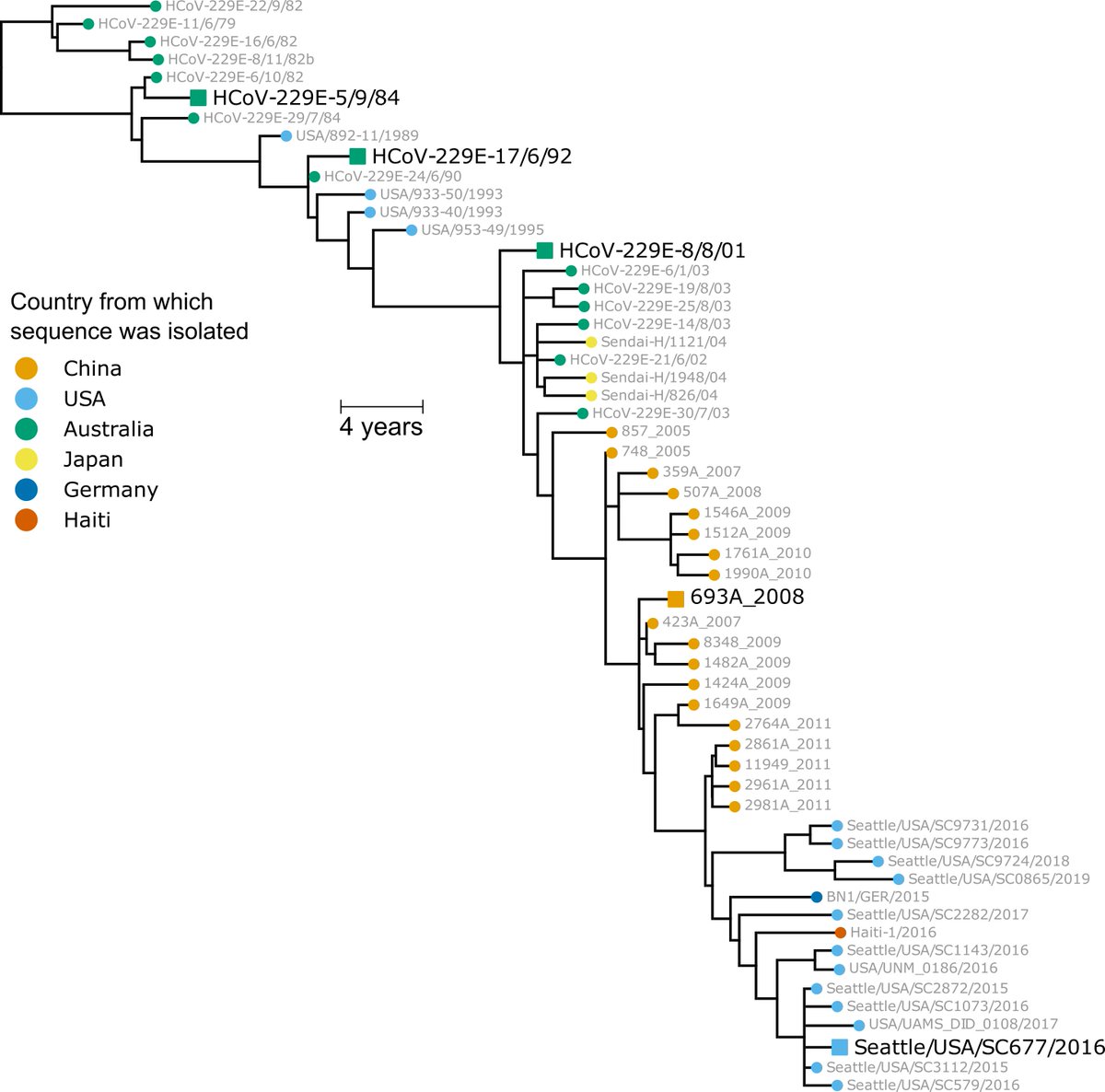
This relative role of RBD & NTD mutations consistent w historical evolution of common-cold CoV-229E, where mutations concentrated in receptor-binding loops of RBD, but also in parts of NTD. Here is plot of mutational variability in CoV-229E spike:
https://twitter.com/jbloom_lab/status/1339939746068353025(15/n)
What do results mean for possible #SARSCoV2 immune escape? Certainly mutations like E484K are concerning. But they *reduce* neut activity, they don't ablate it. Again, look at CoV-229E: takes years of evolution to escape serum neut of most people
https://twitter.com/jbloom_lab/status/1339939732529111040(16/n)
Heterogeneity of mutation effects across sera could be blessing, as mutations that reduce neutralization by some people won't affect others. Look below at how differently evolution of CoV-229E erodes neutralization by different people (Fig 2A of biorxiv.org/content/10.110…) (17/n) 

Heterogeneity also shown by @e_andreano @RommieAmaro @McLellan_Lab, who found multiple mutations that greatly (300-fold) reduce neut of #SARSCoV2 by one human serum but often only modestly (a few fold) affect other sera: biorxiv.org/content/10.110… (18/n)
Scientifically, we see two priorities going forward: (a) Perform similar studies of vaccine sera. Is vaccine immunity affected by mutations similarly to natural immunity? (b) Monitor for antigenic mutations so vaccines can eventually be updated if needed. (19/n)
But biggest priority is vaccinate! Despite above, I'm confident current vaccines will be useful for quite a while. Reasons: (a) even worst mutations (ie, E484) only erode neut activity of some sera, don't eliminate it for any, (b) current vaccines elicit strong immunity... (20/n)
... (c) evidence in animals (& from humans after 1st vaccine dose) that modest immunity can blunt disease, (d) natural immunity to seasonal CoV provides some homologous protection for 3+ years even though they evolve too (see wellcomeopenresearch.org/articles/5-52) (21/n)
Finally, most credit goes to @AllieGreaney, the outstanding grad student who led our study. She worked with Andrea Loes, @khdcrawford, @tylernstarr, & Keara Malone. @HelenChuMD was our invaluable clinical collaborator, and thanks to the volunteers in her HAARVI cohort (22/n)
I'd also like to call out the important work by @houzhou @Tuliodna and cowoerkers in characterizing lineage 501Y.V2 initially identified in South Africa, which carries RBD mutations including E484K, which our study suggests has an antigenic effect: medrxiv.org/content/10.110… (23/n)
Our functional work is potentially useful because the high-quality viral surveillance and rapid reporting of data exemplified by their study.
I'd also like to correct terminology earlier in this Tweet chain. It is lineage 501Y.V2, initially identified in South Africa. (24/n)
I'd also like to correct terminology earlier in this Tweet chain. It is lineage 501Y.V2, initially identified in South Africa. (24/n)
It should be referred by full lineage, not country as in my earlier Tweet. In addition, E484K has been independently observed in viruses from other locations, so any antigenic impacts of this mutation is *not* a concern confined to any country or viral lineage. (25/n)
One more Tweet to share data by @firefoxx66 inspired by useful comments from @Tuliodna. Please read this:
Key implication: the E484K mutation was most prominently reported by @Tuliodna in the 501Y.V2 lineage first identified in South Africa. (26/n)
https://twitter.com/firefoxx66/status/1346546912644759553?s=20
Key implication: the E484K mutation was most prominently reported by @Tuliodna in the 501Y.V2 lineage first identified in South Africa. (26/n)
But importantly, muts at E484 arisen multiple times in several countries over last 9 months. Our functional work (& others cited above) show E484 muts antigenically important. And surveillance by @Tuliodna @rjlessells @atrvlncc & others show this mutation is circulating (27/n)
But because this mutation has arisen multiple times in multiple locations, it is not a concern confined to any particular country. Instead, globally important to continue to monitor evolution, characterize mutations, & assess any eventual implications for vaccine updates. (28/n)
• • •
Missing some Tweet in this thread? You can try to
force a refresh