
Stop tweeting
Start vaccinating
Heading out to vaccinate “people” in Rhode Island today
It may not be a big stadium, it may only be a VFW, it may only be 450 little shots, but these “people” are somebody’s world ... let’s give their community a big shot in the arm
#cmgsays
Start vaccinating
Heading out to vaccinate “people” in Rhode Island today
It may not be a big stadium, it may only be a VFW, it may only be 450 little shots, but these “people” are somebody’s world ... let’s give their community a big shot in the arm
#cmgsays
The COVID vaccination is an intramuscular injection. It is NOT a subcutaneous injection. To give a #COVID19 IM injection DO NOT pinch the skin & Do NOT angle the needle at 45 degrees. This will cause the injection to go into the subcutaneous fat rather than the deltoid muscle. 


To give an IM injection, aim the needle perpendicular to or at 90 degrees to the skin. The CDC does not recommend that you draw back on the needle (there are no big vessels in the deltoid muscle), 
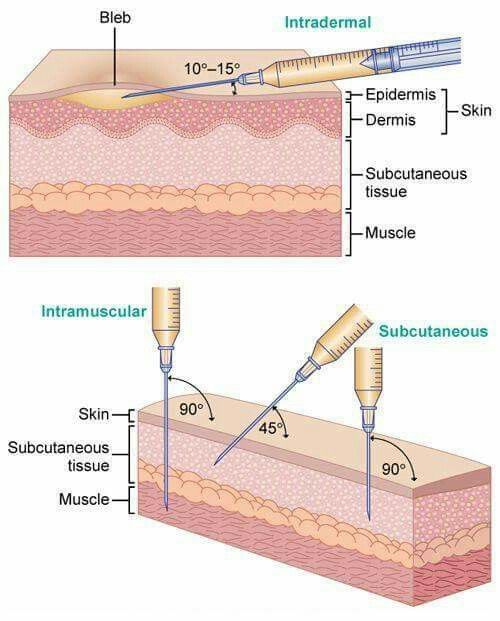
You don't want the vaccine to leak back out of the muscle through the needle track.
So use the Z technique where you pull the skin sideways with the hand you are not injecting with so that when you let go, there is not a straight line from the muscle to leak out of the body.
So use the Z technique where you pull the skin sideways with the hand you are not injecting with so that when you let go, there is not a straight line from the muscle to leak out of the body.

Why give vaccines into the muscle (IM) instead of under the skin (sub q)?
The muscle has more blood vessels & the vaccine gets absorbed into the body better & you are more likely to get an immune response compared with the fatty tissue under the skin. ncbi.nlm.nih.gov/pmc/articles/P…
The muscle has more blood vessels & the vaccine gets absorbed into the body better & you are more likely to get an immune response compared with the fatty tissue under the skin. ncbi.nlm.nih.gov/pmc/articles/P…

There is a temptation to want to massage or rub the vaccinate area after vaccination.
Try to avoid this as it may again squeeze some of the vaccine out of the muscle where all the good blood flow is into the fatty tissue under the skin which does not have as good of blood flow.
Try to avoid this as it may again squeeze some of the vaccine out of the muscle where all the good blood flow is into the fatty tissue under the skin which does not have as good of blood flow.
In summary:
Do Pull the skin sideways
Don't Pinch the skin
Don't Massage the skin
Do Pull the skin sideways
Don't Pinch the skin
Don't Massage the skin
You have now been "armed" for your injection and don't be shy!
If you see someone pinching the skin and injecting at 45 degrees like I have seen on TV, speak up!
Tell them to not pinch and to go in at 90 degrees!
If you see someone pinching the skin and injecting at 45 degrees like I have seen on TV, speak up!
Tell them to not pinch and to go in at 90 degrees!
Use the short needle on the left for smaller people
Use the longer needle on right if if looks like it is a long way to the muscle
Use the longer needle on right if if looks like it is a long way to the muscle

Not a drop of vaccine was wasted yesterday: if people missed their appointment, there was a waiting list of people to call and they came in.
We literally did not have a drop left.
We literally did not have a drop left.
• • •
Missing some Tweet in this thread? You can try to
force a refresh














