💉Biotech Industry Primer💉
A hot nurse stabs one in ur arm. Wohoo! Ur immune to COVID! (ish)
Now how did this lil vax make it from bench to bedside?
👇
1/ Overview
--Pre-launch
2/ FDA stages
3/ Key drivers
--Post-launch
4/ Supply Chain
5/ Trends
--COVID
6/ Price tag
7/ History
A hot nurse stabs one in ur arm. Wohoo! Ur immune to COVID! (ish)
Now how did this lil vax make it from bench to bedside?
👇
1/ Overview
--Pre-launch
2/ FDA stages
3/ Key drivers
--Post-launch
4/ Supply Chain
5/ Trends
--COVID
6/ Price tag
7/ History

1/ US pharma in a nutshell: high capex, highly regulated, long time to profitability.
Recent industry-wide trends (last 5-10yrs):
- Higher cost to bring drugs to market
- Pricing pressure from shift to value-based care
- Decreasing # of late stage pipeline assets
- Consolidation

Recent industry-wide trends (last 5-10yrs):
- Higher cost to bring drugs to market
- Pricing pressure from shift to value-based care
- Decreasing # of late stage pipeline assets
- Consolidation


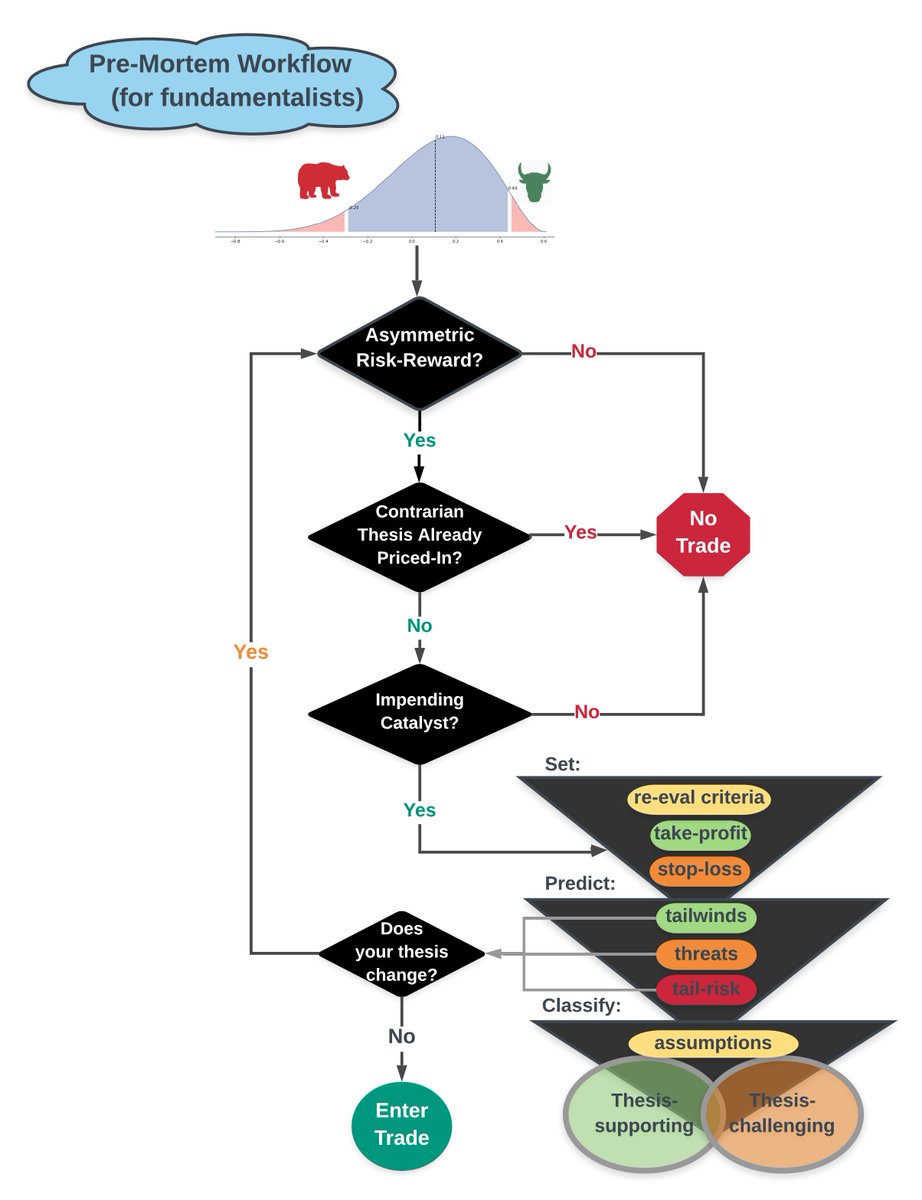
Today, pharma co's face 3 main types of risk.
Scientific
- 12 yrs avg time to market
- only 0.1% of drugs make it to human testing from preclinical
Delivery
- KOLs/marketing
- payer resistance
- govt scrutiny
Economic
- declining ROI
- competition via generics
- patent cliffs
Scientific
- 12 yrs avg time to market
- only 0.1% of drugs make it to human testing from preclinical
Delivery
- KOLs/marketing
- payer resistance
- govt scrutiny
Economic
- declining ROI
- competition via generics
- patent cliffs
Pharma investment specializes into 2 niches (chronologically).
- pre-launch (before drug is sellable) focuses on scientific & clinical analysis
- post-launch (after FDA approval) focuses on commercialization & marketing
Usually, separate analysts cover each niche at a fund.
- pre-launch (before drug is sellable) focuses on scientific & clinical analysis
- post-launch (after FDA approval) focuses on commercialization & marketing
Usually, separate analysts cover each niche at a fund.
Pre- vs. post-launch analysis tracks different catalysts & KPIs.
Pre:
- catalysts are bimodal events around FDA approval (e.g. PDUFA)
- target co’s are startups
Post:
- all about drug adoption curves
- catalysts are M&A & earnings
- diverse target co's (e.g. CMOs, distributors)
Pre:
- catalysts are bimodal events around FDA approval (e.g. PDUFA)
- target co’s are startups
Post:
- all about drug adoption curves
- catalysts are M&A & earnings
- diverse target co's (e.g. CMOs, distributors)
2/ Pre-launch: FDA Stages
Getting to market is entirely about gaining FDA approval.
There are multiple stages: preclinical --> clinical - phases I, II, III --> final review/approval.
Below is a diagram & description of the drug development funnel:

Getting to market is entirely about gaining FDA approval.
There are multiple stages: preclinical --> clinical - phases I, II, III --> final review/approval.
Below is a diagram & description of the drug development funnel:


Preclinical trials: 3+ yrs, testing on lab animals to gather toxicity & efficacy. Must file IND (investigative new drug application) at the end, summarizing preclinical results in order to initiate clinical testing (0.1% get approved).
Clinical trials: 6+ yrs, testing on humans.
Clinical trials: 6+ yrs, testing on humans.
NDAs (new drug applications): submitted after successful human clinical trials, reviewed by AC (advisory committee)
- PDUFA date: deadline for FDA to announce decision
- Usually takes 1-2yrs but can be fast-tracked
- ~6m expedited approval on drugs addressing "high unmet need"
- PDUFA date: deadline for FDA to announce decision
- Usually takes 1-2yrs but can be fast-tracked
- ~6m expedited approval on drugs addressing "high unmet need"
3/ Key Catalysts
- clinical trial results (phases I, II, III)
- AC (advisory) vote results
- PDUFA announcement
- regulatory path clarifications
- "orphan-drug" designation (OD status provides exclusivity for rare disease drugs; benefits: extra 7 yrs of patent protection)
- clinical trial results (phases I, II, III)
- AC (advisory) vote results
- PDUFA announcement
- regulatory path clarifications
- "orphan-drug" designation (OD status provides exclusivity for rare disease drugs; benefits: extra 7 yrs of patent protection)
A biotech investor who wishes to stay anon describes how to analyze clinical trials:
- Trial design: is it double blind or open-label?
- Control treatment: is it placebo or head-to-head comparison vs currently available drug?
- Patient selection: is it the right population?
- Trial design: is it double blind or open-label?
- Control treatment: is it placebo or head-to-head comparison vs currently available drug?
- Patient selection: is it the right population?
- Endpoints (the specific metric being measured btw. control group & test group in the clinical study):
Diff. diseases have diff. endpoints (e.g. AIDS uses amount of HIV in blood). Is the primary endpoint approvable by the FDA? Did they talk to the FDA before starting the trial?
Diff. diseases have diff. endpoints (e.g. AIDS uses amount of HIV in blood). Is the primary endpoint approvable by the FDA? Did they talk to the FDA before starting the trial?
- Statistical significance: Do p-values indicate a high probability that reported endpoint results were not by chance?
- Opinions of KOLs (outside doctors not participating in trial): Is there unmet need? What do they think of the MoA of the drug? If approved, how would they use?
- Opinions of KOLs (outside doctors not participating in trial): Is there unmet need? What do they think of the MoA of the drug? If approved, how would they use?
4/ Post-Market Ecosystem
A drug is FINALLY approved after 10yrs! The original researchers have gone bald. Now what?
Time to make money & reap everything sown/lost to R&D.
Unfortunately (fortunately?) now it's a huge political game: many players with many different incentives.
A drug is FINALLY approved after 10yrs! The original researchers have gone bald. Now what?
Time to make money & reap everything sown/lost to R&D.
Unfortunately (fortunately?) now it's a huge political game: many players with many different incentives.

Here is a flow-diagram showing how each dollar flows through the ecosystem as a drug travels from manufacturer to patient: 

Let's look at each component in depth:
Manufacturers split into:
- "Big Pharma" (e.g. Pfizer, Eli Lily, Merck): mostly rely on M&A of small biotechs to fill their asset pipelines rather than in-house R&D, often outsource manufacturing to CMOs
- CMOs: 3rd party manufacturers
Manufacturers split into:
- "Big Pharma" (e.g. Pfizer, Eli Lily, Merck): mostly rely on M&A of small biotechs to fill their asset pipelines rather than in-house R&D, often outsource manufacturing to CMOs
- CMOs: 3rd party manufacturers
- Wholesale/Distributors: hyper consolidated, basically tri-opoly (CAH, ABC, MCK); brokers that maintain relationships w/ both manufacturers & retail pharmacies/hospitals
- Revenues driven by service fees & discounts/rebates from manufacturer for prompt payment & bulk purchasing
- Revenues driven by service fees & discounts/rebates from manufacturer for prompt payment & bulk purchasing
- Pharmacies: account for 75% of the prescription drug market. (non-retail providers e.g. hospitals, HMOs, clinics, nursing homes, etc. comprise 25%)
Increasingly consolidated niche. Tp 5 pharmacies accounted for >70% of prescription revenues.
Increasingly consolidated niche. Tp 5 pharmacies accounted for >70% of prescription revenues.

- PBMs (pharmacy benefit managers): negotiate discounts & rebates w/ manufacturers on behalf of clients (health plan sponsors)
Also super consolidated (notice a pattern much?): top 3 PBMs manage benefits for 70% of health plans. Also highly "integrated" w/ rest of supply chain.
Also super consolidated (notice a pattern much?): top 3 PBMs manage benefits for 70% of health plans. Also highly "integrated" w/ rest of supply chain.

Now how is each $1 split across & passed around the ecosystem?
Who gets the best bang for the least amount of sweat? Who has the highest margins?
Who wins when healthcare costs skyrocket past sustainability?
Here are 3 hypothetical transactions that dig under the hood:


Who gets the best bang for the least amount of sweat? Who has the highest margins?
Who wins when healthcare costs skyrocket past sustainability?
Here are 3 hypothetical transactions that dig under the hood:



5/ Industry Trends
1. Personalized medicine getting hotter; big pharma M&A eyes gene therapy
2. Regulations driving value-based pricing (mostly in an effort to lower prices)
3. Cooling of M&A from 2015 days (after death of cross-border tax inversion)
4. SPACs looking to de-spac
1. Personalized medicine getting hotter; big pharma M&A eyes gene therapy
2. Regulations driving value-based pricing (mostly in an effort to lower prices)
3. Cooling of M&A from 2015 days (after death of cross-border tax inversion)
4. SPACs looking to de-spac
6/ And the biggest trend of all: COVID
Don't know how mRNAs work. Ask the brilliant @enhancerleo.
But I can tell u abt the HUGE gap btw what countries are paying for the vax.
Pfizer: UK £15 per jab; Belgium £10.6; Israel £34
Moderna: US $15, EU $18
AZ: UK £1.56; S. Africa: £3.8
Don't know how mRNAs work. Ask the brilliant @enhancerleo.
But I can tell u abt the HUGE gap btw what countries are paying for the vax.
Pfizer: UK £15 per jab; Belgium £10.6; Israel £34
Moderna: US $15, EU $18
AZ: UK £1.56; S. Africa: £3.8
For funzies let's toast to more political leadership faux pas besides Trump:
https://twitter.com/JorgeLiboreiro/status/1339897763996332032
7/ COVID Vax History
It's incredible that in less than 1.5 yrs, we've gone from panic, FUD, & global outbreak to 50% of the US (131M ppl) fully-vaxed.
This timeline from Moderna is not only an educational case study showing the FDA stages but a trophy of medical progress.
It's incredible that in less than 1.5 yrs, we've gone from panic, FUD, & global outbreak to 50% of the US (131M ppl) fully-vaxed.
This timeline from Moderna is not only an educational case study showing the FDA stages but a trophy of medical progress.

Huge shout-out to my real-life friend @black6cap for sharing his insights & notes!! I know nothing. He's the guru! 😀🧠
• • •
Missing some Tweet in this thread? You can try to
force a refresh