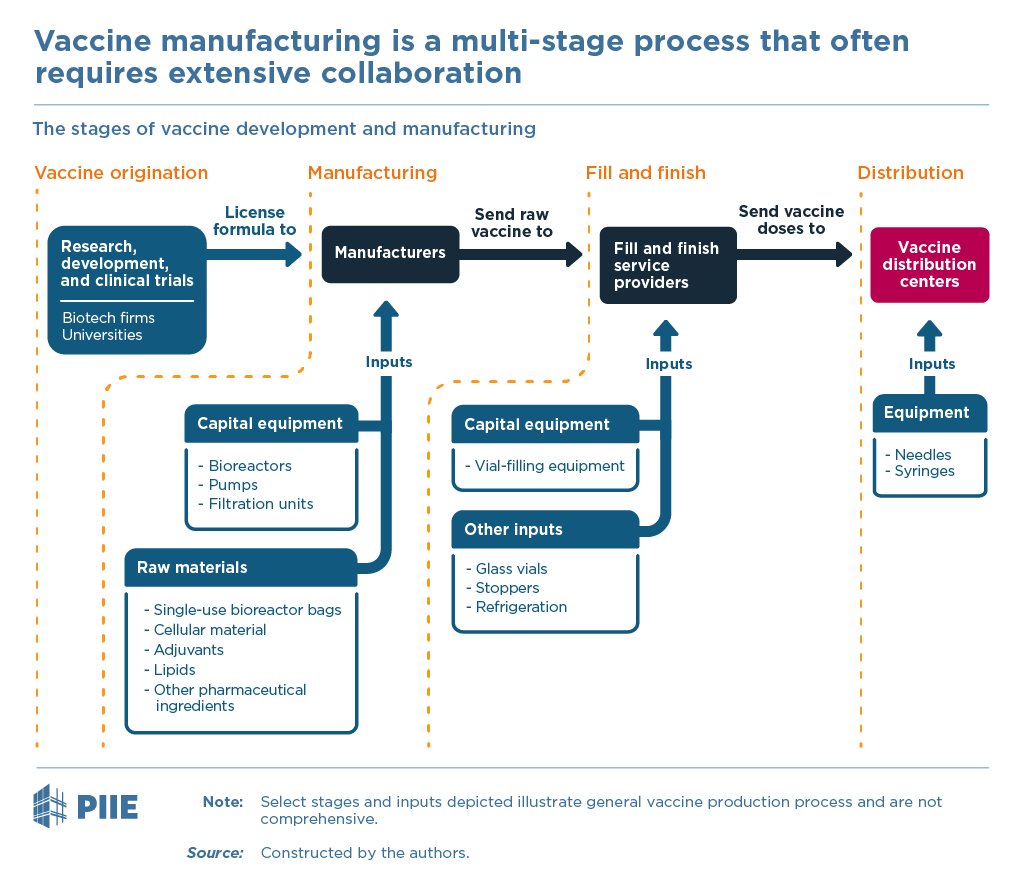
US government was accused of banning exports of vaccine-making supplies, most notably to India. New supply chain data reveals there was never a US export ban. But the episode highlights a problem demanding new policy.
My latest, with @ChrisGHRogers 🧵 1/
piie.com/blogs/trade-an…
My latest, with @ChrisGHRogers 🧵 1/
piie.com/blogs/trade-an…
India is being devastated by the pandemic right now. It needs more vaccines at home, and it needs equipment to make more vaccines for home... 2/
nytimes.com/article/india-…
nytimes.com/article/india-…
Worse, Indian companies had also been expected to play a major role in providing billions of vaccine doses to poor countries globally through Covax.
Hopes were high as late as February 2021. By March, hopes were dashed, and the Serum Institute stopped exporting... 3/

Hopes were high as late as February 2021. By March, hopes were dashed, and the Serum Institute stopped exporting... 3/


Inputs to make vaccines are in short supply globally. [More on contributing causes why below]
But this tweet by Adar Poonawalla, CEO of @SerumInstIndia brought things to a head with the accusation of a US "embargo" on vaccine-making supplies... 4/
But this tweet by Adar Poonawalla, CEO of @SerumInstIndia brought things to a head with the accusation of a US "embargo" on vaccine-making supplies... 4/
https://twitter.com/adarpoonawalla/status/1382978713302683653?s=20
NEW DATA: There was never a US export embargo to the Serum Institute of India.
US exports were up 30% between October 2020 and March 2021 (relative to prior 6 months), including from vaccine input suppliers (ABEC, Merck Millipore, Cytiva, Pall, Thermo Fisher, Sartorius)... 5/
US exports were up 30% between October 2020 and March 2021 (relative to prior 6 months), including from vaccine input suppliers (ABEC, Merck Millipore, Cytiva, Pall, Thermo Fisher, Sartorius)... 5/

Similarly, Biological E, with a license to manufacture both Johnson & Johnson (Janssen) vaccine and the candidate from Baylor/Dynavax (neither yet authorized by Indian regulators, a separate issue) had also made complaints of US export restrictions... 6/
ft.com/content/7225cb…
ft.com/content/7225cb…
NEW DATA: There was never a US ban on exports to Biological E either. US exports to Biological E between October 2020 and March 2021 were 220% higher than the previous 6 months, also from major US-based suppliers of vaccine equipment and materials... 7/ 

Note: the US policy response in April was to send immediate vaccine-making supplies to India... 8/
https://twitter.com/JakeSullivan46/status/1386359529865162752?s=20
But we need to get to the source of the shortage problem of vaccine-making equipment and material inputs, and deploy POLICY to make sure *THAT* does not hold back global vaccine production.
So what is the problem?
Making vaccines requires A LOT of specialized inputs... 9/
So what is the problem?
Making vaccines requires A LOT of specialized inputs... 9/

Serum Institute is producing the Oxford/AstraZeneca vaccine.
@CliveHGlover a scientist at Pall, one of the key equipment providers, explained the benefit of using the SAME inputs at all of the AstraZeneca sites:
🤓👉🏾 pall.com/en/biotech/blo… ... 10/
@CliveHGlover a scientist at Pall, one of the key equipment providers, explained the benefit of using the SAME inputs at all of the AstraZeneca sites:
🤓👉🏾 pall.com/en/biotech/blo… ... 10/

Standardizing AstraZeneca production would have tradeoffs.
[+ ,😀] speed at which each new facility could scale up production of a consistent drug product.
[- ,☹️] it may concentrate—and lock in—demand for equipment into a limited number of suppliers... 11/
[+ ,😀] speed at which each new facility could scale up production of a consistent drug product.
[- ,☹️] it may concentrate—and lock in—demand for equipment into a limited number of suppliers... 11/
PROBLEM: Serum Institute is competing with A LOT of other production facilities globally to buy that same equipment to start making the Oxford / AstraZeneca vaccine
Benefits of using the same inputs are only realized if there is enough standardized equipment to go around... 12/
Benefits of using the same inputs are only realized if there is enough standardized equipment to go around... 12/

Same for Serum Institute complaints about needing equipment and raw materials to manufacture the Novavax vaccine.
If that equipment is common across Novavax plants, Serum Institute is competing for the same materials from the same few supplier companies at the same time.... 13/
If that equipment is common across Novavax plants, Serum Institute is competing for the same materials from the same few supplier companies at the same time.... 13/

A NEW AND GLOBAL COVID-19 VACCINE SUPPLY CHAIN POLICY IS NEEDED
Much of the uncertainty is now gone.
Dozens of facilities making vaccines have been established globally. Trade data shows their relationships with suppliers.
Policymakers: LEVERAGE that information... 14/
Much of the uncertainty is now gone.
Dozens of facilities making vaccines have been established globally. Trade data shows their relationships with suppliers.
Policymakers: LEVERAGE that information... 14/
FIVE STEP PLAN
1. Regularly survey the dozens of now-established COVID-19 vaccine production facilities about their critical inputs.
How much do they need, from which companies, from where are those being supplied, and on what time schedule? 15/
1. Regularly survey the dozens of now-established COVID-19 vaccine production facilities about their critical inputs.
How much do they need, from which companies, from where are those being supplied, and on what time schedule? 15/
2. Once that information has been collected for each facility, aggregate it up to the level of the input supplier.
How many bioreactor bags are needed from Cytiva versus Thermo Fisher. How many filters are needed from Merck Millipore, etc. 16/
How many bioreactor bags are needed from Cytiva versus Thermo Fisher. How many filters are needed from Merck Millipore, etc. 16/
3. Separately survey the major input suppliers identified in step 2.
Cross-check whether each input supplier’s pending orders match the information from the vaccine facilities. 17/
Cross-check whether each input supplier’s pending orders match the information from the vaccine facilities. 17/
4. Identify potential input shortages.
Whenever step 3 reveals a supplier as not having sufficient capacity to meet all of the demand on time, some sort of policy intervention is needed. 18/
Whenever step 3 reveals a supplier as not having sufficient capacity to meet all of the demand on time, some sort of policy intervention is needed. 18/
5. Determine whether the shortage is impacting a customized input, and tailor the policy response accordingly
Customized...
...Short-run: incentivize wartime-like-effort, running second, third, weekend shifts.
...Long-run: incentivize investment needed to expand capacity 19/
Customized...
...Short-run: incentivize wartime-like-effort, running second, third, weekend shifts.
...Long-run: incentivize investment needed to expand capacity 19/
5. (cont) Determine whether the shortage is impacting a customized input, and tailor the policy response accordingly
NON-customized inputs:
Use the step 3 information to help find temporary alternative suppliers. 20/
NON-customized inputs:
Use the step 3 information to help find temporary alternative suppliers. 20/
US policymakers did some of the 5-step process for US vaccine makers through Operation Warp Speed and the Defense Production Act.
The Indian experience shows why the US can not and should not attempt to manage this five-step process alone. 22/
The Indian experience shows why the US can not and should not attempt to manage this five-step process alone. 22/
Transparency in the global vaccine supply chain will also improve trust. That may heighten cooperation and be a reminder that, in the COVID-19 pandemic, we are all in this together.
(comments welcome) ENDS/
piie.com/blogs/trade-an…
(comments welcome) ENDS/
piie.com/blogs/trade-an…
• • •
Missing some Tweet in this thread? You can try to
force a refresh