[Thread] 1. How does @SAHPRA1 operate?
Helen Rees, board chair:
Governing Acts require Saphra to be:
1. Transparent, fair and objective
2. Allow Sahpra to co-operate with other regulatory authorities
3. Evaluate applications of all medical devices
Helen Rees, board chair:
Governing Acts require Saphra to be:
1. Transparent, fair and objective
2. Allow Sahpra to co-operate with other regulatory authorities
3. Evaluate applications of all medical devices

2. @sahpra is an autonomous entity:
1. Sahpra reports to parliament, the health minister and the Sahpra board
2. The health min appoints Sahpra's board
3. The CEO makes operational decisions, not the board
1. Sahpra reports to parliament, the health minister and the Sahpra board
2. The health min appoints Sahpra's board
3. The CEO makes operational decisions, not the board

3. Boitumelo Semete-Makokotlela, @SAHPRA1 CEO: Sahpra is internationally recognised for its stringent standards. Part of the reason why the WHO has chosen SA to host the new #mRNA tech transfer hub (for #COVID vaccines), is because of the detailed, rigorous processes of Sahpra. 

4. @SAHPRA1 helped to investigate the fake vaccines which entered the country last year from China and they worked with the police to stop the falsified jabs from being distributed in SA. The occurrence of fake medicines in SA is lower than in many other countries.
5. What does the CEO do? The CEO doesn't make decisions alone. There is extensive consultation with academics and scientists. If an applicant isn't satisfied with a @sahpra decision, they can appeal. First to the CEO and then to the health minister. 

6. @sahpra is part of very few African regulators who are part of an international coalition of medicines regulators such as the FDA, TGA (Australia), EMA (Europe) and NAFDAC (Nigeria) to collaborate on #COVID19 product approvals. They share data and advice. 

7. @SAHPRA1 doesn't procure medicines, that's the role of the public/private healthcare system. Sahpra's job is to establish whether those products are safe + whether they do what their manufacturers say they do. Once approved, both the private + public health sectors can use it.
8. These are the steps @SAHPRA1 follows in order to establish if a product such as a #COVIDVaccine is safe and effective. Sahpra also looks at manufacturing processes after it's approved a product: if production processes are not sound post approval, they can revoke an approval. 

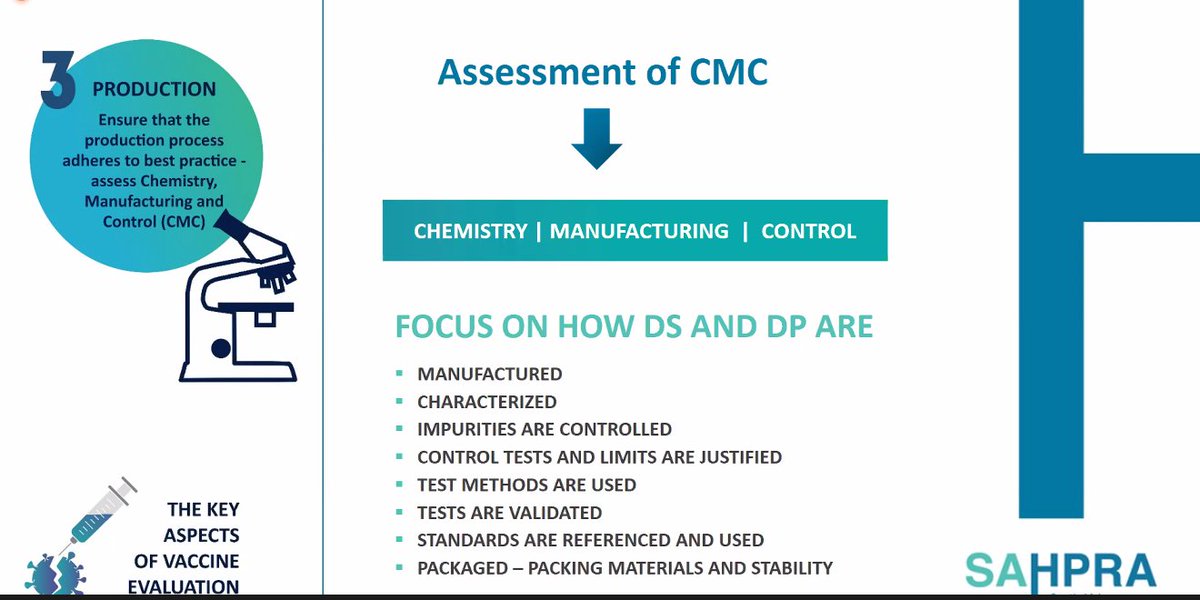
9. @SAHPRA1 assesses whether there are impurities in products to establish if they're safe. They also look at how products are packaged. 
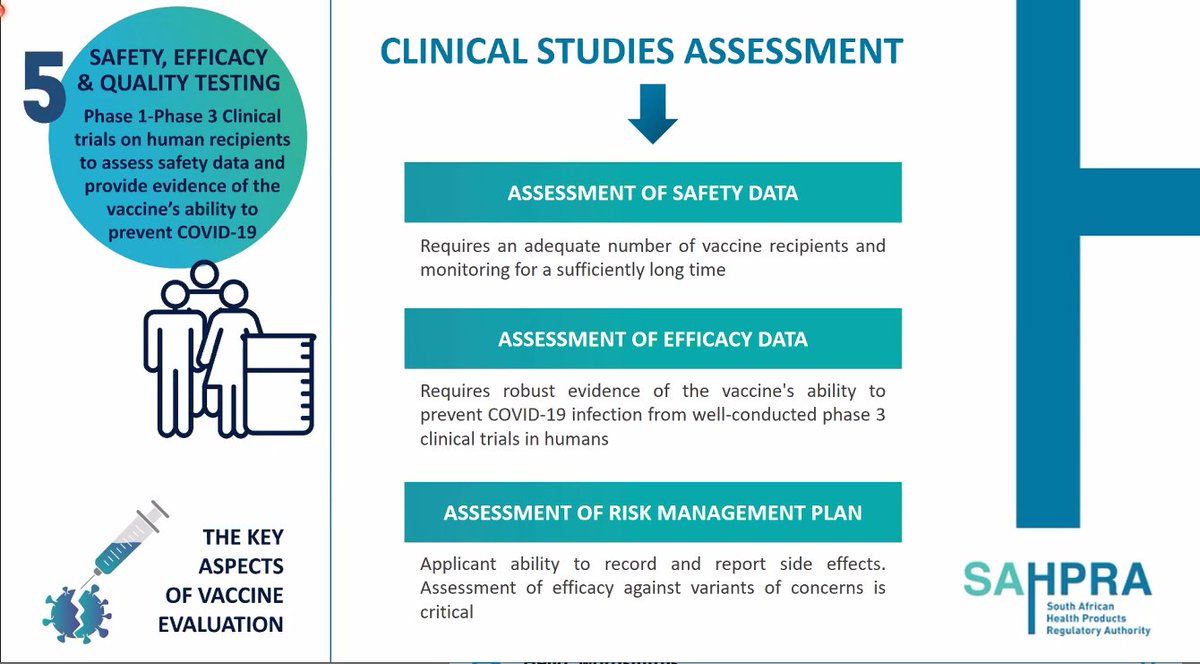
10. @SAHPRA1 looks at Phase 3 trial data that has been submitted for a product such as a #COVIDVaccine. It reassess the raw data and the way efficacy has been calculated. 
11. With regards to #COVID19, @SAHPRA1 doesn't just assess vaccines. It is currently evaluating 50+ antibody COVID tests and it has overseen the approval of PPE equipment such as specialised masks, and also ventilators and treatments such as dexamethasone. 

12. @SAHPRA1 can withdraw product approvals. In the case of Section 21 (emergency use authorisation) approvals (most #COVID vaccines are approved this way), it can withdraw approvals immediately. For market authorisations, there's a longer process.
13. Normally, @sahpra takes about 20 months to approve a vaccine. For #COVID19 jabs, that process has been sped up to about 90 work days.
• • •
Missing some Tweet in this thread? You can try to
force a refresh