1/
Nephrology:
“We will forever argue about validity of equations to convert serum creatinine to eGFR using age, sex etc”
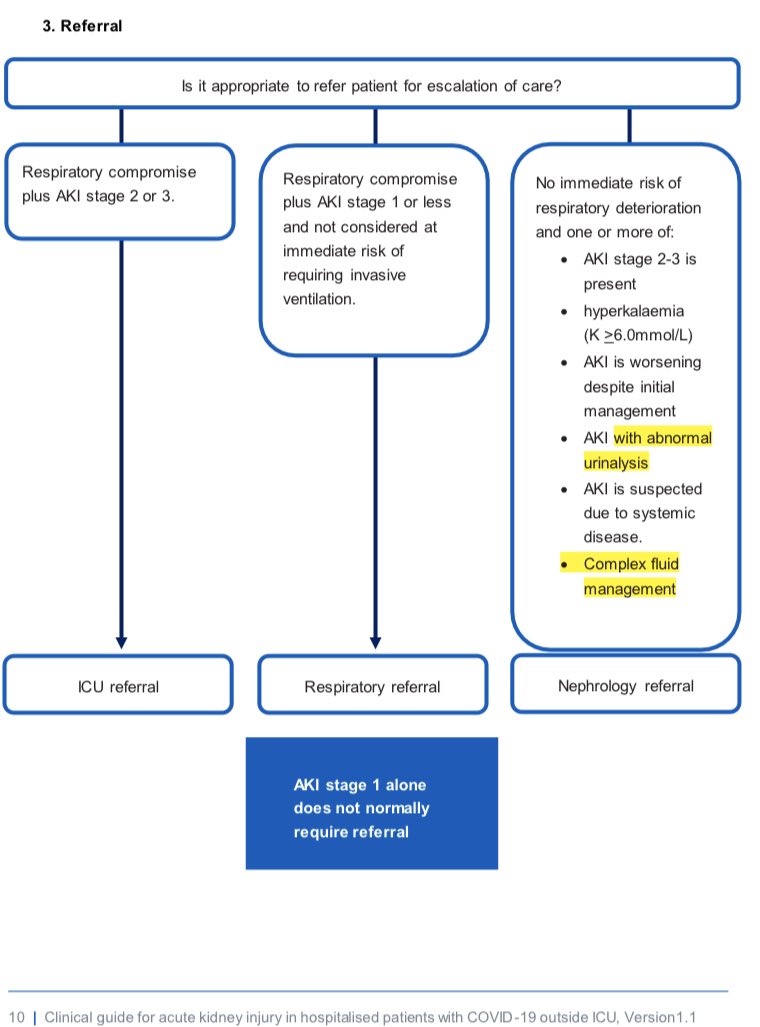
Also nephrology:
“We will make no adjustment for muscle mass when using urinary creatinine within urine ACR”
A #tweetorial on two albuminuria paradoxes 👇
Nephrology:
“We will forever argue about validity of equations to convert serum creatinine to eGFR using age, sex etc”
Also nephrology:
“We will make no adjustment for muscle mass when using urinary creatinine within urine ACR”
A #tweetorial on two albuminuria paradoxes 👇

2/
Recap: why use early morning urine ACR?
✅Correlates well with ‘gold standard’ 24hr urine collection (pain to perform)
✅Creatinine in denominator corrects for urine dilution - works when creat excretion constant (but, note some daily variation)
ncbi.nlm.nih.gov/pmc/articles/P…
Recap: why use early morning urine ACR?
✅Correlates well with ‘gold standard’ 24hr urine collection (pain to perform)
✅Creatinine in denominator corrects for urine dilution - works when creat excretion constant (but, note some daily variation)
ncbi.nlm.nih.gov/pmc/articles/P…

3/
Albuminuria quantification is useful prognostically (as well as clear diagnostic utility!):
❗️Independent risk factor for CV mortality
❗️ RENAAL showed higher uACR = higher risk of CKD progression in patients with type 2 diabetes
pubmed.ncbi.nlm.nih.gov/15302780/
Albuminuria quantification is useful prognostically (as well as clear diagnostic utility!):
❗️Independent risk factor for CV mortality
❗️ RENAAL showed higher uACR = higher risk of CKD progression in patients with type 2 diabetes
pubmed.ncbi.nlm.nih.gov/15302780/

4/
But enter PARADOX 1️⃣…
Urinary creatinine excretion is proportional to muscle mass, just like serum creatinine is.
So why does the uACR value not undergo extensive adjustment like eGFR does, based on studies looking to adjust for patient body surface area, age, sex, etc?
But enter PARADOX 1️⃣…
Urinary creatinine excretion is proportional to muscle mass, just like serum creatinine is.
So why does the uACR value not undergo extensive adjustment like eGFR does, based on studies looking to adjust for patient body surface area, age, sex, etc?
5/
(It’s worth noting that the heavy-lifting of converting serum creatinine to eGFR is done by indexing to body surface area (BSA), with age & sex (rather imperfectly) doing fine-tuning.
ajkd.org/article/S0272-…
But we don’t even standardise uACR to BSA, let alone age or sex.)
(It’s worth noting that the heavy-lifting of converting serum creatinine to eGFR is done by indexing to body surface area (BSA), with age & sex (rather imperfectly) doing fine-tuning.
ajkd.org/article/S0272-…
But we don’t even standardise uACR to BSA, let alone age or sex.)
6/
Does this matter? Sometimes!
e.g. in males (so therefore ⬆️muscle mass & ⬆️urinary creatinine denominator) with diabetes, spot uACR gave much lower results than when albuminuria measured by timed urine collection.
ncbi.nlm.nih.gov/pmc/articles/P…
Does this matter? Sometimes!
e.g. in males (so therefore ⬆️muscle mass & ⬆️urinary creatinine denominator) with diabetes, spot uACR gave much lower results than when albuminuria measured by timed urine collection.
ncbi.nlm.nih.gov/pmc/articles/P…
7/
On flip side, raised uACR can mark low muscle mass rather than large albumin excretion, due to a lower urinary creatinine denominator.
Therefore therapies by uACR threshold (RAASi, SGLT2i, etc) are targeted more at those with lower muscle mass (on average older, female pts).
On flip side, raised uACR can mark low muscle mass rather than large albumin excretion, due to a lower urinary creatinine denominator.
Therefore therapies by uACR threshold (RAASi, SGLT2i, etc) are targeted more at those with lower muscle mass (on average older, female pts).
8/
This graph shows that heavier & younger patients need higher actual 24 hour albuminuria to reach the old KDOQI threshold for uACR “microalbuminuria” - so they’d miss out on earlier treatment with RAASi.
karger.com/Article/Fullte…
This graph shows that heavier & younger patients need higher actual 24 hour albuminuria to reach the old KDOQI threshold for uACR “microalbuminuria” - so they’d miss out on earlier treatment with RAASi.
karger.com/Article/Fullte…

9/
However, is there any advantage to this sloppy approach?
Well - spot uACR performs BETTER than 24hr urine albumin collection in predicting CKD progression!
ncbi.nlm.nih.gov/pmc/articles/P…
However, is there any advantage to this sloppy approach?
Well - spot uACR performs BETTER than 24hr urine albumin collection in predicting CKD progression!
ncbi.nlm.nih.gov/pmc/articles/P…

10/
It’s because uACR is a bipolar tool – it captures ⬆️protein leak AND ⬇️muscle mass, & both are negative prognostic factors.
This is an occasion when ‘surrogate test' performs BETTER than ‘gold standard’ test. Can anyone think of other examples of this in medicine? I can’t
It’s because uACR is a bipolar tool – it captures ⬆️protein leak AND ⬇️muscle mass, & both are negative prognostic factors.
This is an occasion when ‘surrogate test' performs BETTER than ‘gold standard’ test. Can anyone think of other examples of this in medicine? I can’t
11/
These principles also apply to urine Protein-Creatinine ratio (uPCR) but we’ll stick with discussing uACR for now.
If you want to recap the evidence for converting between these measures see the great #NephJC blogpost from @sophia_kidney
nephjc.com/news/acrpcr
These principles also apply to urine Protein-Creatinine ratio (uPCR) but we’ll stick with discussing uACR for now.
If you want to recap the evidence for converting between these measures see the great #NephJC blogpost from @sophia_kidney
nephjc.com/news/acrpcr
12/
Now let’s also think about uACR PARADOX 2️⃣!
Patient 1 – GFR 120, weight 50kg, loses 1g albuminuria per 24 hours, uACR 70mg/mmol
Patient 2 – GFR 40, weight 50kg, loses 1g albuminuria per 24 hours, uACR 70mg/mmol
How does their glomerular permeability compare?
Now let’s also think about uACR PARADOX 2️⃣!
Patient 1 – GFR 120, weight 50kg, loses 1g albuminuria per 24 hours, uACR 70mg/mmol
Patient 2 – GFR 40, weight 50kg, loses 1g albuminuria per 24 hours, uACR 70mg/mmol
How does their glomerular permeability compare?
13/
They have the same urine ACR & 24hr albumin leak, but (simplistically speaking) patient 2 only has 1/3 the nephrons, so therefore each individual glom is 3 times leakier!
This will have prognostic and therapeutic implications - but this isn’t factored in by using uACR.
They have the same urine ACR & 24hr albumin leak, but (simplistically speaking) patient 2 only has 1/3 the nephrons, so therefore each individual glom is 3 times leakier!
This will have prognostic and therapeutic implications - but this isn’t factored in by using uACR.
14/
At 1g/day albuminuria this may not be treatment altering, but at lower levels and when ‘labelling’ patients as having CKD, it is potentially problematic.
Therefore, logically, when calculating uACR we should factor in the nephron mass across which albumin is lost.
At 1g/day albuminuria this may not be treatment altering, but at lower levels and when ‘labelling’ patients as having CKD, it is potentially problematic.
Therefore, logically, when calculating uACR we should factor in the nephron mass across which albumin is lost.
15/
So what could be done differently?
We could all divide uACR by the eGFR, and use that number instead.
This would:
🧩Provide some adjustment for muscle mass, to address Paradox 1
🧩Account for size of functioning nephron mass, to adjust for Paradox 2
So what could be done differently?
We could all divide uACR by the eGFR, and use that number instead.
This would:
🧩Provide some adjustment for muscle mass, to address Paradox 1
🧩Account for size of functioning nephron mass, to adjust for Paradox 2
16/
Evidence that this approach would do anything to improve patient care is absent - but at least the world would be logical???
Further reading for those interested:
karger.com/Article/Fullte…
Evidence that this approach would do anything to improve patient care is absent - but at least the world would be logical???
Further reading for those interested:
karger.com/Article/Fullte…
17/
So why don’t we bother?
✅ Variability is high between different uACR samples within individuals
✅ Importance of trends > absolute values
✅ Few clinical diagnoses or decisions hinge on small differences
So why don’t we bother?
✅ Variability is high between different uACR samples within individuals
✅ Importance of trends > absolute values
✅ Few clinical diagnoses or decisions hinge on small differences
18/
But just in case you had arbitrary cut-offs in mind like:
“I would biopsy this woman as her uACR is 110mg/mmol, but not this man whose uACR is only 80mg/mmol”
bear in mind the latter might just have the greater albumin leak!
But just in case you had arbitrary cut-offs in mind like:
“I would biopsy this woman as her uACR is 110mg/mmol, but not this man whose uACR is only 80mg/mmol”
bear in mind the latter might just have the greater albumin leak!
Take homes:
👍Spot uACR can often replace 24hr urine collection
👍Be mindful that ANY urine ratio test with creatinine as denominator will underestimate in more muscular & overestimate in sarcopaenia
👍 Having said that - uACR is a great prognostic test, do send it & use it!
👍Spot uACR can often replace 24hr urine collection
👍Be mindful that ANY urine ratio test with creatinine as denominator will underestimate in more muscular & overestimate in sarcopaenia
👍 Having said that - uACR is a great prognostic test, do send it & use it!
Thanks to @amyaimei @Nephro_Sparks @drM_sudha @docanjuyadav for their comments and feedback, and to @EllamTim (sadly not a twitter fan) for lots of teaching on the topic!
• • •
Missing some Tweet in this thread? You can try to
force a refresh