Can we outsmart #cancer and stop it before it even starts?
Our brand new paper🔥@NatureComms reveals a novel stem-like cell population directly related to #breast tumor initiation.
Let's dig in🧵🧵
Our brand new paper🔥@NatureComms reveals a novel stem-like cell population directly related to #breast tumor initiation.
Let's dig in🧵🧵

First, quick background.
Sadly, everybody reading this knows breast cancer.
It is the most commonly diagnosed cancer in women, with a staggering 1 in every 8 women in the world receiving this diagnosis throughout their lifetimes.
Sadly, everybody reading this knows breast cancer.
It is the most commonly diagnosed cancer in women, with a staggering 1 in every 8 women in the world receiving this diagnosis throughout their lifetimes.
Multiple factors have been shown to modulate breast cancer risk.
You might already know that:
An active lifestyle🏃♀️, a good diet 🥦 or breastfeeding 🤱 are protective, while high breast density, radiation exposure or hormone replacement therapy are detrimental.
You might already know that:
An active lifestyle🏃♀️, a good diet 🥦 or breastfeeding 🤱 are protective, while high breast density, radiation exposure or hormone replacement therapy are detrimental.

Unfortunately, what you might not know is that the absolute risk reduction from such protective lifestyle factors is depressingly modest.
But, make no mistake and prepare to be shocked😳.
Breast cancer is one of the very few cancers that can be effectively prevented!
But, make no mistake and prepare to be shocked😳.
Breast cancer is one of the very few cancers that can be effectively prevented!
Enter Pregnancy🤰
A single full-term early pregnancy (< 25 yrs) decreases the lifelong risk of hormone receptor positive breast cancer (85% of all breast cancers) by an unbelievable 40%.
Later first pregnancies (> 30 yrs) are still protective, but less so.
A single full-term early pregnancy (< 25 yrs) decreases the lifelong risk of hormone receptor positive breast cancer (85% of all breast cancers) by an unbelievable 40%.
Later first pregnancies (> 30 yrs) are still protective, but less so.

Unfortunately, in practice, preventing breast cancer is not that easy. On the contrary.
We all know that full-term early pregnancies are not feasible as a population-level breast cancer prevention strategy.
We all know that full-term early pregnancies are not feasible as a population-level breast cancer prevention strategy.
There’s more:
The lifelong breast cancer risk of BRCA1/2 germline mutation carriers 🧬 is even further increased‼️ by pregnancies, reaching an unbelievable 80% for BRCA1+ carriers with one child.
The lifelong breast cancer risk of BRCA1/2 germline mutation carriers 🧬 is even further increased‼️ by pregnancies, reaching an unbelievable 80% for BRCA1+ carriers with one child.

The natural question now becomes:
Can we mimic the protective effect of early🤰with a 💊?
Let's try!
But in order to do this, we need to first map out the mechanisms by which pregnancy decreases breast cancer risk.
Can we mimic the protective effect of early🤰with a 💊?
Let's try!
But in order to do this, we need to first map out the mechanisms by which pregnancy decreases breast cancer risk.
Past data @LabPolyak shows that p27+ (CDKN1B+) progenitors with high TGFB pathway activity are decreased after pregnancy.
Therefore, TGFB could regulates the proliferation & pool size of hormone-responsive mammary epithelial progenitors which are cell of origin in breast cancer.
Therefore, TGFB could regulates the proliferation & pool size of hormone-responsive mammary epithelial progenitors which are cell of origin in breast cancer.

If so,modulating the TGFB pathway could modulate breast cancer risk.
Let's test this.
1.Strategy🧭
Deplete p27+ progenitors via inhibition of TGFB. Decreasing these progenitors could prevent tumors.
2.Intervention⚡️
Short treatment with TGFB Receptor1 inhibitor in 2 rat models
Let's test this.
1.Strategy🧭
Deplete p27+ progenitors via inhibition of TGFB. Decreasing these progenitors could prevent tumors.
2.Intervention⚡️
Short treatment with TGFB Receptor1 inhibitor in 2 rat models
In a mind-boggling experiment, after treating rats with the inhibitor (TGFBRi) for 10 days, we expose them to a potent carcinogen.
90% of untreated rats develop tumors, whereas only 40% of treated rats do!
‼️In other words: 50% of rats are tumor-free because of the treatment😯
90% of untreated rats develop tumors, whereas only 40% of treated rats do!
‼️In other words: 50% of rats are tumor-free because of the treatment😯

Digging deeper:
1. The treated rats have fewer tumors which develop later.
2. The mammary glands of tumor-free animals are histologically normal.
Hence: This treatment 💊 has a long-term cancer preventive effect without perturbing normal mammary physiology.
Why is this?
1. The treated rats have fewer tumors which develop later.
2. The mammary glands of tumor-free animals are histologically normal.
Hence: This treatment 💊 has a long-term cancer preventive effect without perturbing normal mammary physiology.
Why is this?
Enter molecular #scRNAseq data 🧬
We find that the TGFBRi treatment promotes epithelial features (shifted keratin distribution), and that all 3 main breast mammary gland epithelial populations (basal, luminal progenitor, mature luminal) are heavily perturbed by the treatment.
We find that the TGFBRi treatment promotes epithelial features (shifted keratin distribution), and that all 3 main breast mammary gland epithelial populations (basal, luminal progenitor, mature luminal) are heavily perturbed by the treatment.

Surprisingly, in the single cell data, the TGFBRi treatment induces cell cycle proliferation of progenitors, as shown by unique groups of cells with strong expression of proliferation and cell-cycle markers popping up after treatment. 

The most striking treatment-induced change is how a totally novel population with both epithelial basal & secretory features emerges right after treatment.
We term them Secretory Basal Cells (SBCs).
We identify SBCs in both rat strains and create a SBC characteristic signature.
We term them Secretory Basal Cells (SBCs).
We identify SBCs in both rat strains and create a SBC characteristic signature.

We characterize SBCs in great detail, via experiments & computational analyses.
SBCs have strong mammary epithelial stem cell features. They are enriched in Terminal End Buds, a specialized structure consisting of stem cells & guiding pubertal mammary development & cancer risk.
SBCs have strong mammary epithelial stem cell features. They are enriched in Terminal End Buds, a specialized structure consisting of stem cells & guiding pubertal mammary development & cancer risk.

Again surprisingly, SBCs are enriched in tissues from the mammary gland of high-risk conditions (women without any pregnancy-nulliparous vs. women with pregnancies-parous), as well as in tissues from BRCA1/2+ carriers.
Hence,SBCs are positively associated with breast cancer risk
Hence,SBCs are positively associated with breast cancer risk

Interactome analysis shows a central role for SBCs in the mammary epithelium.
SBCs are the most active cell type (highest number of connections/cell) and they most actively regulate insulin-IGF signaling, a critical pathways for mammary gland development & breast cancer risk.
SBCs are the most active cell type (highest number of connections/cell) and they most actively regulate insulin-IGF signaling, a critical pathways for mammary gland development & breast cancer risk.

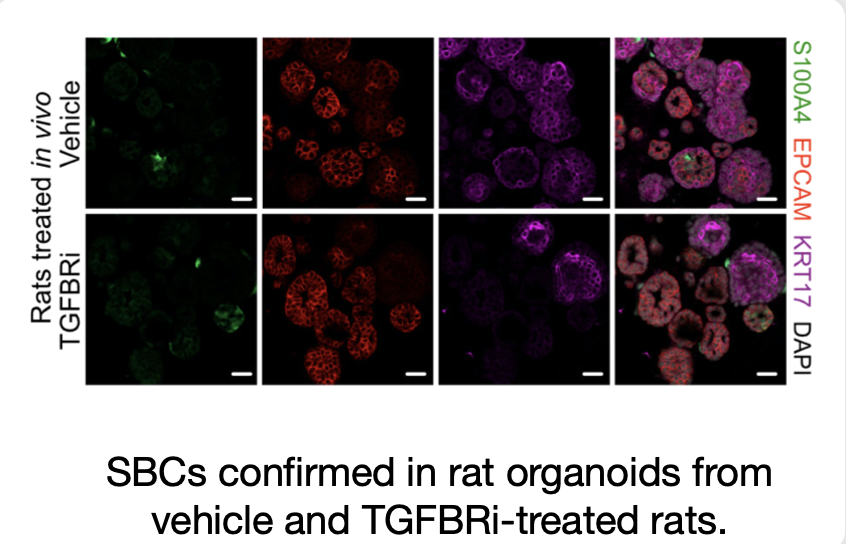
Finally, we also validate the epithelial nature and presence of the SBC subpopulation experimentally in rat organoid data from both control and TGFBRi-treated rats.
In treated rats, this population is expanded, confirming SBC enrichment following treatment.
In treated rats, this population is expanded, confirming SBC enrichment following treatment.
Let's recap📚
1. A novel breast epithelial population (SBC) emerges right after a TGFBRi short-term treatment which prevents tumors in 2 rat models.
2. SBCs are associated with increased proliferation.
3. SBCs are stem-like.
4. SBCs are enriched in high-risk cancer conditions
1. A novel breast epithelial population (SBC) emerges right after a TGFBRi short-term treatment which prevents tumors in 2 rat models.
2. SBCs are associated with increased proliferation.
3. SBCs are stem-like.
4. SBCs are enriched in high-risk cancer conditions
Taken together, these observations seem counter-intuitive🤔
But, biology is complex. What we believe is actually happening:
Treatment💊
⬇️
Transient increase📈in progenitor proliferation
⬇️
Long-term depletion of the progenitor pool
⬇️
Lifelong decrease📉in breast cancer risk
But, biology is complex. What we believe is actually happening:
Treatment💊
⬇️
Transient increase📈in progenitor proliferation
⬇️
Long-term depletion of the progenitor pool
⬇️
Lifelong decrease📉in breast cancer risk

Take home messages‼️
1. Breast cancer is one of the few cancers for which a potent natural prevention strategy does exist.
Understanding the underlying mechanisms by which this happens is an immense opportunity still hidden in plain sight.
1. Breast cancer is one of the few cancers for which a potent natural prevention strategy does exist.
Understanding the underlying mechanisms by which this happens is an immense opportunity still hidden in plain sight.
2. Breast cancer can be prevented by targeting TGFB-responsive mammary epithelial progenitors in 2 different rat models.
This offers preclinical evidence for the design of breast cancer prevention strategies initially targeting women at high risk of developing breast cancer 🧬
This offers preclinical evidence for the design of breast cancer prevention strategies initially targeting women at high risk of developing breast cancer 🧬
However, our results are still very preliminary.
There are immense challenges ahead on the way to clinical implementation⛰️
We’re thinking about these issues a lot and doing follow-up studies and experiments.
There are immense challenges ahead on the way to clinical implementation⛰️
We’re thinking about these issues a lot and doing follow-up studies and experiments.
We believe cancer prevention is the future.
Cancers are evolutionary beasts. Fighting them is hard. Current cancer treatments are complicated & often don't work.
This is why stopping cancer before it starts is the way forward.
This is real & possible.
Cancers are evolutionary beasts. Fighting them is hard. Current cancer treatments are complicated & often don't work.
This is why stopping cancer before it starts is the way forward.
This is real & possible.
All #Bioinformatics #DataScience code and materials to support these results is on GitHub github.com/csimona/tumor-…
The raw and preprocessed genomics data is on GEO 0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/geo/query/acc.…
Preprocessed #RStats objects can be downloaded from Zenodo zenodo.org/record/7293642…
The raw and preprocessed genomics data is on GEO 0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/geo/query/acc.…
Preprocessed #RStats objects can be downloaded from Zenodo zenodo.org/record/7293642…
Finally, here is the link to the @NatureComms paper. Read it and get it touch!
This was a monumental effort spanning many years together with @LabPolyak @nellage & so many wonderful colleagues @DanaFarber @harvardmed @dfcidatascience
Thank you all!
nature.com/articles/s4146…
This was a monumental effort spanning many years together with @LabPolyak @nellage & so many wonderful colleagues @DanaFarber @harvardmed @dfcidatascience
Thank you all!
nature.com/articles/s4146…
• • •
Missing some Tweet in this thread? You can try to
force a refresh