COVID-Out double-blind randomized trial tested #metformin, ivermectin, or fluvoxamine for outpatient #COVID19. Secondary endpoint was incidence of Long Covid (PASC).
Volunteers were followed monthly to 10 mo.
Metformin 42% reduction in Long Covid.
medrxiv.org/content/10.110…
Volunteers were followed monthly to 10 mo.
Metformin 42% reduction in Long Covid.
medrxiv.org/content/10.110…
Incidence of Healthcare provider diagnosed long covid (PASC) was 42% less among those randomized to #metformin vs. matched placebo.
Hazard Ratio for Long Covid in the metformin group versus control was 0.58 (95% CI 0.38 to 0.88, P=0.009);
Hazard Ratio for Long Covid in the metformin group versus control was 0.58 (95% CI 0.38 to 0.88, P=0.009);

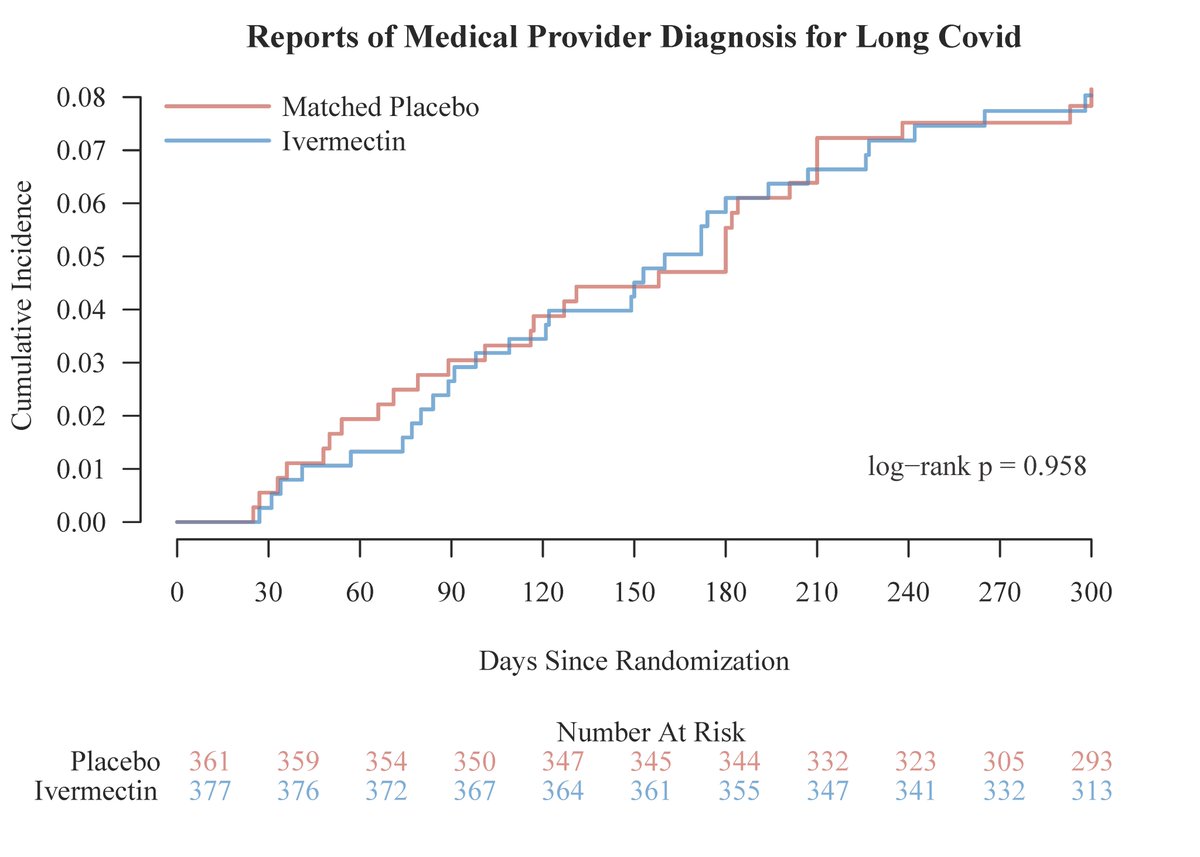
There was no benefit for those randomized to #ivermectin vs. matched placebo for reducing long covid and low-dose #fluvoxamine was not beneficial either for decreasing #longcovid. 


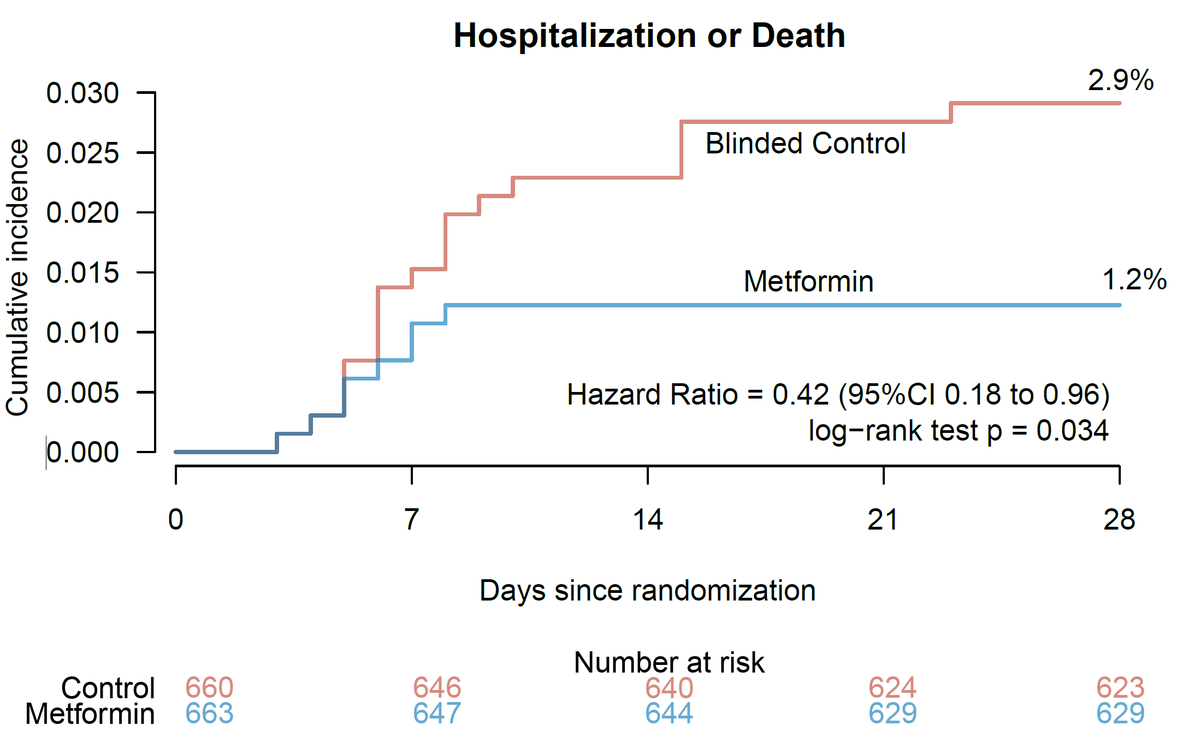
In the original trial, there was a 42% reduction in ER visits & hospitalizations through 14 days.
28-day risk of hospitalization/death was also decreased. 1.34% (8/596) of those receiving metformin were hospitalized or died compared to 3.16% (19/601) of blinded controls (P=.034)
28-day risk of hospitalization/death was also decreased. 1.34% (8/596) of those receiving metformin were hospitalized or died compared to 3.16% (19/601) of blinded controls (P=.034)
Initial trial focused on 14-day outcomes after COVID.
Guidelines committee correctly pointed out that there was not a reduction in 14-day hospitalizations in the mITT population but ignored the statistical difference in the ITT population or at 28-days nejm.org/doi/full/10.10…
Guidelines committee correctly pointed out that there was not a reduction in 14-day hospitalizations in the mITT population but ignored the statistical difference in the ITT population or at 28-days nejm.org/doi/full/10.10…
Cumulative incidence of Healthcare provider diagnoses of Long Covid for #metformin. Statistically significant by univariate or adjusted analysis (P=0.009). 

The metformin effect at prevention of Long Covid was generally consistent across subgroups and across variant time periods.
(In general, I would not over interpret these smaller subgroups)
(In general, I would not over interpret these smaller subgroups)

Metformin
Absolute risk reduction was 4.4% (95%CI, 1.1% to 7.6%).
Number needed to treat to prevent 1 Long Covid case was 23 (95%CI, 13 to 92).
At med cost $0.54 for a 14-day course of immediate release metformin (n=36 tabs), cost per case averted is $12.30 (95%CI, $7 to $49).
Absolute risk reduction was 4.4% (95%CI, 1.1% to 7.6%).
Number needed to treat to prevent 1 Long Covid case was 23 (95%CI, 13 to 92).
At med cost $0.54 for a 14-day course of immediate release metformin (n=36 tabs), cost per case averted is $12.30 (95%CI, $7 to $49).
What are strengths?
1. Randomized
2. Blinded (participants, healthcare providers, investigators, and outcomes assessors)
3. Large Sample size, n=1125
4. Lost to follow up ~10% through 6mo.
5. Across multiple variant periods w/ & w/o vaccination
1. Randomized
2. Blinded (participants, healthcare providers, investigators, and outcomes assessors)
3. Large Sample size, n=1125
4. Lost to follow up ~10% through 6mo.
5. Across multiple variant periods w/ & w/o vaccination
What are limitations?
1. Healthcare provider diagnosis.
2. Changing nature of the definition of Long Covid (What is Long COVID?)
3. Trial excluded low-risk individuals by excluding those with a normal BMI <25 and age <30, thus may not be generalizeable to these groups.
1. Healthcare provider diagnosis.
2. Changing nature of the definition of Long Covid (What is Long COVID?)
3. Trial excluded low-risk individuals by excluding those with a normal BMI <25 and age <30, thus may not be generalizeable to these groups.
Randomized and blinded are HUGE strengths as some of the non-specific, non-COVID related things that occur would be equally randomized between groups.
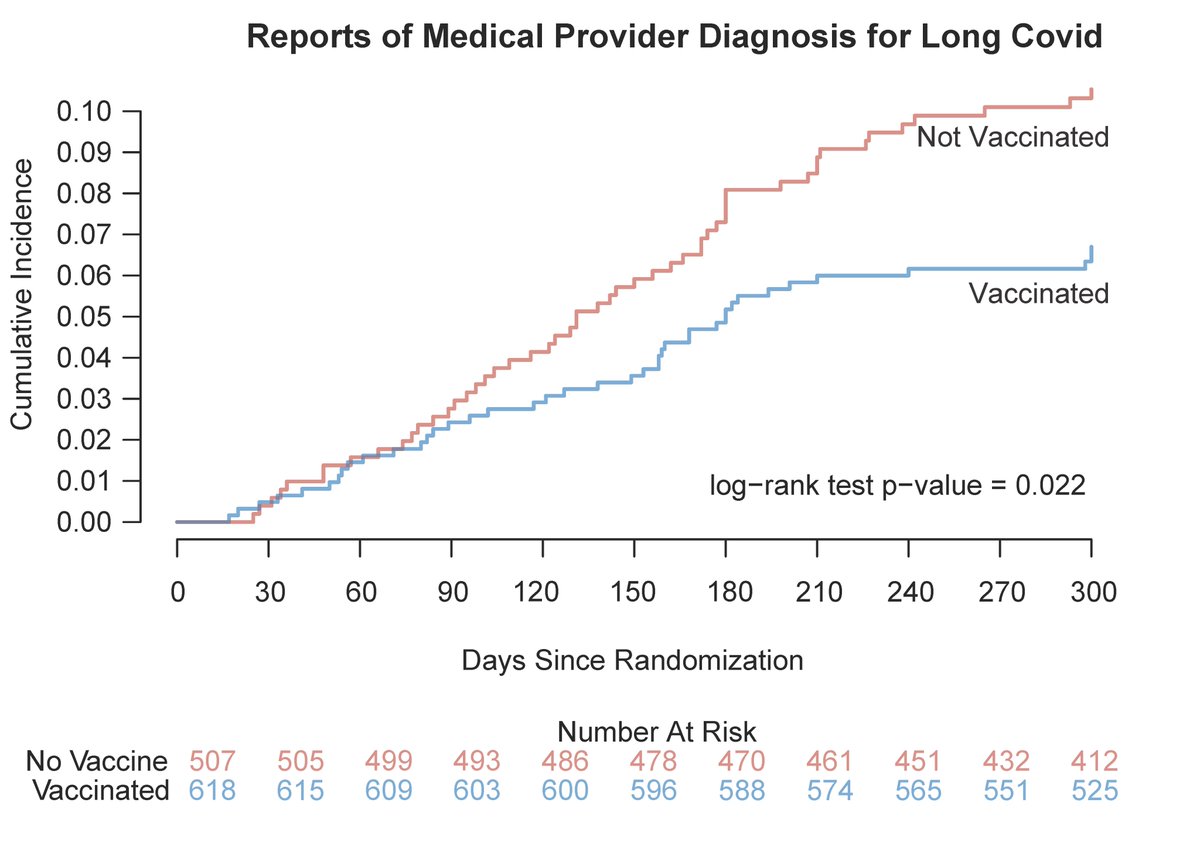
Those vaccinated had less #LongCovid than those unvaccinated.
Assessing vaccine was not the purpose of the trial, and this is not a randomized comparison, so view this as a prospective observational cohort.
Cumulative incidence of when medical provider made the LC diagnosis
Assessing vaccine was not the purpose of the trial, and this is not a randomized comparison, so view this as a prospective observational cohort.
Cumulative incidence of when medical provider made the LC diagnosis
What's metformin's mechanism of action? Probably antiviral, likely some anti-inflammatory.
There are 8+ studies looking at metformin in vitro activity.
pubmed.ncbi.nlm.nih.gov/?term=metformi…
Covid-Out collected viral swabs at Day 1, 5, 10.
Pre-print upcoming.
There are 8+ studies looking at metformin in vitro activity.
pubmed.ncbi.nlm.nih.gov/?term=metformi…
Covid-Out collected viral swabs at Day 1, 5, 10.
Pre-print upcoming.
Metformin may have a role in other RNA viral infections and is being studied in TB as an immodumodulatory drug.
I purposely say "may" as metformin should be studied.
I purposely say "may" as metformin should be studied.
https://twitter.com/PaulSaxMD/status/1606717646141296641
Demographics were similar between those who developed long covid vs did not. Those with long COVID had slightly higher BMI, more likely unvaccinated, less boosted, and more freq women (although 89% of ♀️ did not develop long covid), 

An obvious question, what is the mechanism of #metformin in #covid19?
MET has substantial antiviral properties.
Multiple in vitro lab studies.
More data coming.
May likely have immunomodulatory effects too, but I can't prove that.
Note:
Substantial is a deliberate word choice.
MET has substantial antiviral properties.
Multiple in vitro lab studies.
More data coming.
May likely have immunomodulatory effects too, but I can't prove that.
Note:
Substantial is a deliberate word choice.
• • •
Missing some Tweet in this thread? You can try to
force a refresh