Another confirmatory trial of beneficial effect of #fluvoxamine 100mg BID with Budesonide inhaler in a highly vaccinated population for #COVID19
Dose matters. 50mg 2x/d is not 100mg 2x/d. FLV 50mg 2x/d did not have benefit in two trials
Dose matters. 50mg 2x/d is not 100mg 2x/d. FLV 50mg 2x/d did not have benefit in two trials
https://twitter.com/ABsteward/status/1648074491573026817
As a combo blinded trial (FLV + inhaler), which one had benefit? Hard to know. In activ-6, we did not observe a benefit of inhaled fluticasone in a double blind RCT, yet UK Principle did see a benefit in open label trial with budesonide.
Importantly the #fluvoxamine dose studied here was 100mg 2x/day.
Covid-out and activ-6 both confirmed a lack of benefit of 50mg 2x/day dose.
If I gave someone 1/2 the effective dose of an HIV med & it didn't work, does that mean the med doesn't work? Or just dose was wrong?
Covid-out and activ-6 both confirmed a lack of benefit of 50mg 2x/day dose.
If I gave someone 1/2 the effective dose of an HIV med & it didn't work, does that mean the med doesn't work? Or just dose was wrong?
What does this RCT mean?
In the US, it will be ignored as preventing vaccinated people from deteriorated to needing to go to the ER or being hospitalized is not viewed as beneficial to people -- per @US_FDA and NIH guidelines panel.
Do we not believe double blind RCTs anymore?
In the US, it will be ignored as preventing vaccinated people from deteriorated to needing to go to the ER or being hospitalized is not viewed as beneficial to people -- per @US_FDA and NIH guidelines panel.
Do we not believe double blind RCTs anymore?
Instead of RCTs for expensive on-patent drug(s) that fails to show any benefit in a vaccinated population in a randomized clinical trial (EPIC-SR, Panoramic), we get guidelines and FDA labels for treatment of expansive non-studied populations based on case-control studies
Yet such a combo therapy is potentially important in low and middle income countries (LMIC)
Second basic scientists should be investigating the sigma-1 pathway that fluvoxamine dampens... as a possible sepsis therapy.
ncbi.nlm.nih.gov/pmc/articles/P…
Second basic scientists should be investigating the sigma-1 pathway that fluvoxamine dampens... as a possible sepsis therapy.
ncbi.nlm.nih.gov/pmc/articles/P…
Fluvoxamine is not a perfect drug. It has drug-drug interactions, including with CAFFEINE. (Avoid caffeine).
Tolerability is a problem as 20-25% can't tolerate FLV.
But that receptor and pathway should be explored further for better host directed therapies.
Tolerability is a problem as 20-25% can't tolerate FLV.
But that receptor and pathway should be explored further for better host directed therapies.
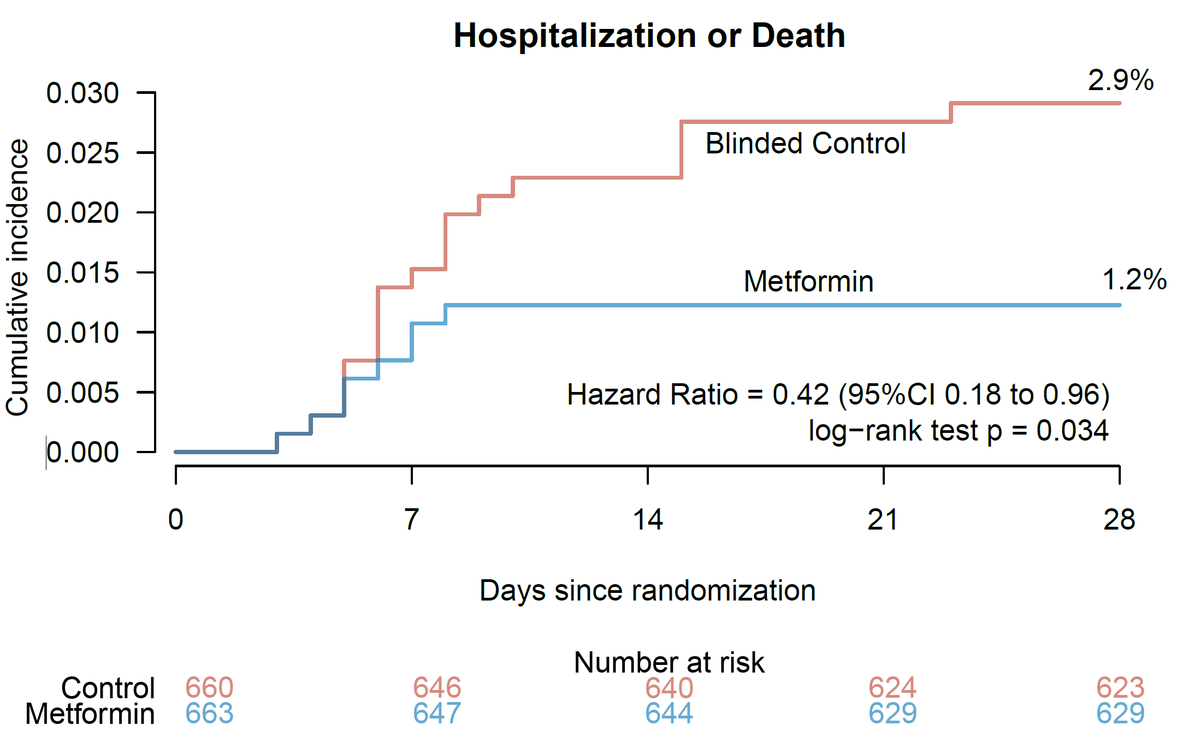
Overall, for #covid19, I still think #metformin remains a good option. Low cost. Reduced 28-day risk of hospitalization by 57% and reduced risk of #longcovid by 41%.
Personally, I am not worried about being hospitalized, but I don't want long covid.
No paxlovid DDI
Personally, I am not worried about being hospitalized, but I don't want long covid.
No paxlovid DDI
In a perfect world, I'd love to see a 2x2 factorial trial testing paxlovid vs. Metformin in a vaccinated population powered toward reduction of #longcovid incidence at 90 days.
Paxlovid + metformin could be a very interesting combo.
Paxlovid + metformin could be a very interesting combo.
As above, this combo of FLV+ budesonide inhaler, I think is most relevant for LMIC settings for #covid19 but #metformin is another option as well (which I like better). Having options that MDs can discuss with patients is better than @WHO recommending unavailable therapies.
Overall, it's rather pointless to do randomized trials for #covid19 anymore, unless one is looking at trying to prevent #LongCovid or targeting immunocompromised persons who remain at risk.
Otherwise one has to run ~26000 person trials to show reduction in hospitalization.
Otherwise one has to run ~26000 person trials to show reduction in hospitalization.
But ACTIV-6 trial continues to enroll to look at whether medicines shorten duration of illness in a double blind trial format. Do people feel better faster? Activ6study.org
Do people feel better faster? Continues to be a relevant question.
Yet even if a medicine is shown to be effective at reducing symptoms, some will say -- well it doesn't reduce hospitalizations. If something reduces hospitalizations, then some will say -- it doesn't reduce death
Yet even if a medicine is shown to be effective at reducing symptoms, some will say -- well it doesn't reduce hospitalizations. If something reduces hospitalizations, then some will say -- it doesn't reduce death
Yet despite the nilism that some feel towards low cost repurposed medications, we slog onwards. ACTIV-6 currently is testing montelukast, a common asthma medicine to see if it reduce symptoms faster than placebo.
• • •
Missing some Tweet in this thread? You can try to
force a refresh