Have you ever wondered why patients in shock are refractory to vasopressor support if also severely acidotic?
I always assumed it was b/c cellular stuff just doesn't work well at low pH.
It turns out the answer is slightly more complicated...
#medtwitter #tweetorial
A similarly diminished response was observed for human skeletal muscles when stimulated with phenylephrine at varying pH's
bit.ly/2oVCdYt
To understand why vasopressors don't work as well under acidotic conditions we need to remind ourselves what determines blood pressure, aka Ohm's law:
❤️Cardiac output x systemic vascular resistance = blood pressure
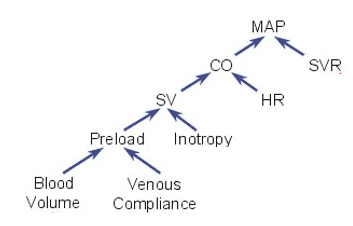
As we all learned in physiology class, cardiac output is determined by heart rate, stroke volume, and inotropy (as well as adequate preload).
bit.ly/2BmWgBN
It turns out that reducing pH INSIDE a cardiac myocyte (and vascular smooth cell) has several downstream effects on adrenergic signalling:
👎 Opening of K exporter channels -> hyperpolarization of the cell membrane ->closing of Ca importer channels
bit.ly/2qiOWor
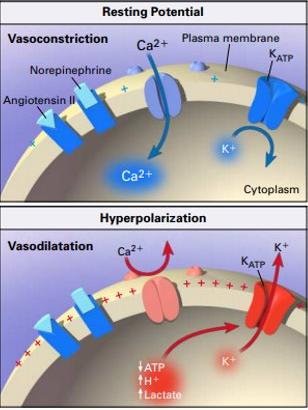
Reduced Ca influx into cells leads to:
👎Reduced binding of calcium to myofibrils
👎Vasodilation of vascular smooth muscle cells and decreased inotropy of cardiac myocytes
This = refractoriness to vasopressor stimulation at low intracellular pH.
bit.ly/31u066C
But what about the impact of pH OUTSIDE the cell?
Amazingly, extracellular acidosis causes a decrease in the amount of cell-surface beta-adrenergic receptors (at least in ex vivo chick embryo ventricular cells)
bit.ly/35OnXRR
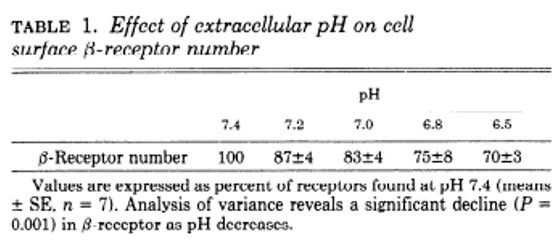

How/why this occurs is unknown but may be due to phosphorylation and subsequent sequestration of the receptor.
bit.ly/2MpGj45

A natural follow-up question would be if there is a particular serum pH below which vasopressors stop working well?
The answer is we don't know, but the above literature suggested a drop off in response at pH < 7.2
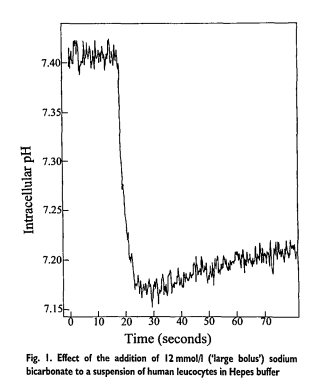
Another follow-up question is, if vasopressors don't work well with severe acidosis, should we use alkaline infusions (such as sodium bicarbonate/HCO3) to raise the pH?
This is actually controversial because HCO3 actually DECREASES intracellular pH (probably by being converted to CO2 and creating an H+ ion when reacting with intracellular water).
As we learned before, not something you want to do
bit.ly/2J1e6P9
Let's sum up what we've learned:
💡Response to vasopressors⬇️w/ acidosis
💡Intracellular acidosis leads to⬆️K efflux, hyperpolarization,⬇️Ca influx into cardiac/vascular muscle cells
💡Extracellular acidosis also⬇️surface adrenergic receptors
💡HCO3⬇️cell pH = controversial




















