Should we use #hydroxychloroquine (HCQ) +/- azithromycin to treat #COVID19 #SARSCoV2? Let’s take a look at the data.
Here’s: #HowIReadThisPaper on the only trial published to date on HCQ for COVID19 in humans:
Gautret et al: mediterranee-infection.com/wp-content/upl…
(Thread)

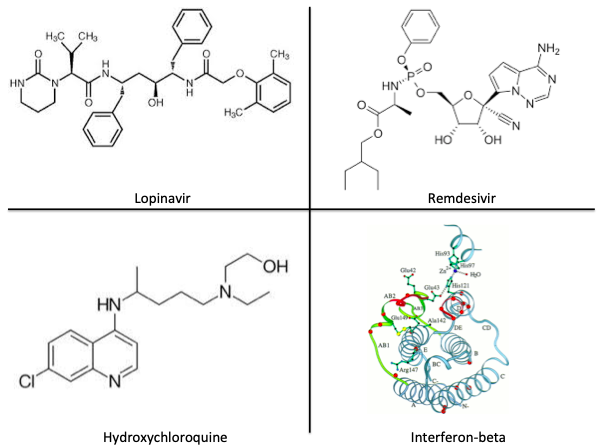
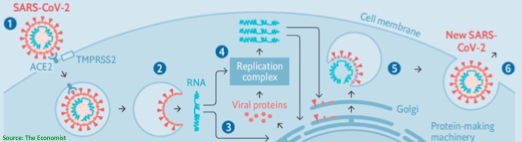
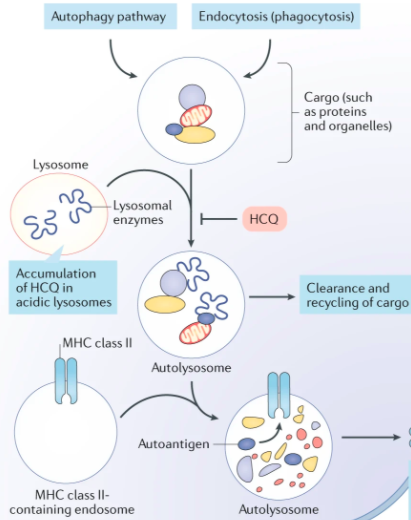
Background: HCQ has antiviral activity against SARS-CoV-1 in vitro, is already available in much of the world, and has a known side-effect profile. It was used for #COVID19 in China, but data on clinical outcomes are not yet available.
Question: Does HCQ shorten the duration of viral shedding in patients with #COVID19?
Date Published: 17 March 2020 (In Press)
Funding: Agence Nationale de la Recherche (Government of France)
Study Design: Open-label (unblinded) non-randomized clinical trial, also known as a prospective cohort study
Population: 42 patients with PCR-confirmed #COVID19 in France were included; 26 received HCQ, 16 received usual care
Study Period: The first half of March, 2020
Intervention: HCQ 200 mg PO TID x10 days +/- azithromycin (to prevent bacterial superinfection) at the discretion of treating clinicians
Control: Usual care (without HCQ or azithromycin)
Study Procedures: HCQ was offered to patients at one study site. Patients who consented = intervention group, patients who refused or were admitted at the other three study sites = control group.
Inclusion Criteria: Hospitalized patients aged >12 years, NP swab + for #SARSCoV2 on admit, able to provide informed consent
Exclusion Criteria: Allergy or contraindication to HCQ (retinopathy, G6PD deficiency, or prolonged QT interval), pregnant or breastfeeding women
Protocol available? Yes: clinicaltrialsregister.eu/ctr-search/sea…
Primary outcome: Percentage of patients with detectable viral shedding on day 6
Secondary outcomes: Time to normalization of T and RR, LOS, and mortality
Duration of follow-up: 14 days
Loss to follow-up: 6/26 (23%) patients receiving HCQ +/- azithromycin; 0/20 (0%) patients receiving usual care
Author’s conclusions: HCQ + azithromycin is associated with a reduction in viral shedding at day 6 in hospitalized patients with #COVID19 compared with usual care.
My appraisal: This study was “positive,” meaning it showed that HCQ was associated with a ~58% reduction in the proportion of patients with any viral shedding at day 6. What are the limitations that threaten the validity of this conclusion?
Lack of blinding and lack of randomization in this study are serious threats to internal validity because they influence allocation of patients and assessment of the outcome. In this study, they are both likely to bias towards a benefit of HCQ +/- azithromycin.
Additionally, among the 6 HCQ patients who dropped out, the authors report that 4 of them were still PCR-positive at the time of dropout. This is likely to bias the results towards a benefit of HCQ.
Finally, the primary endpoint is a surrogate endpoint, and none of the clinical secondary endpoints specified are reported. This is called selective outcome reporting, or outcome reporting bias.
One caveat: because of the small size, short duration, and limited clinical assessment, if there are other real benefits of HCQ +/- azithro in #COVID19, it does not seem likely that this study would have identified them.
Bottom line:
This study is at high risk of bias. Due to small size, lack of randomization or blinding, and multiple sources of bias towards a benefit of HCQ, this evidence should be considered low quality. Better-designed RCTs are needed (and are in process).
(End)