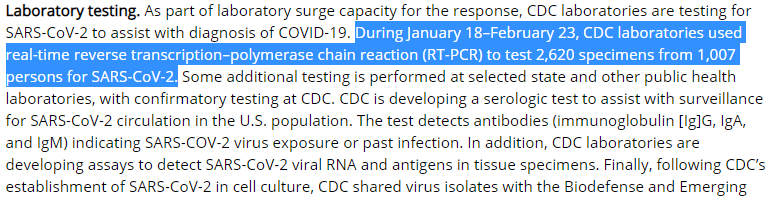
BUT IT'S ACTUALLY WORSE THAN THIS bc these numbers =/= *clinical* sensitivity 1/
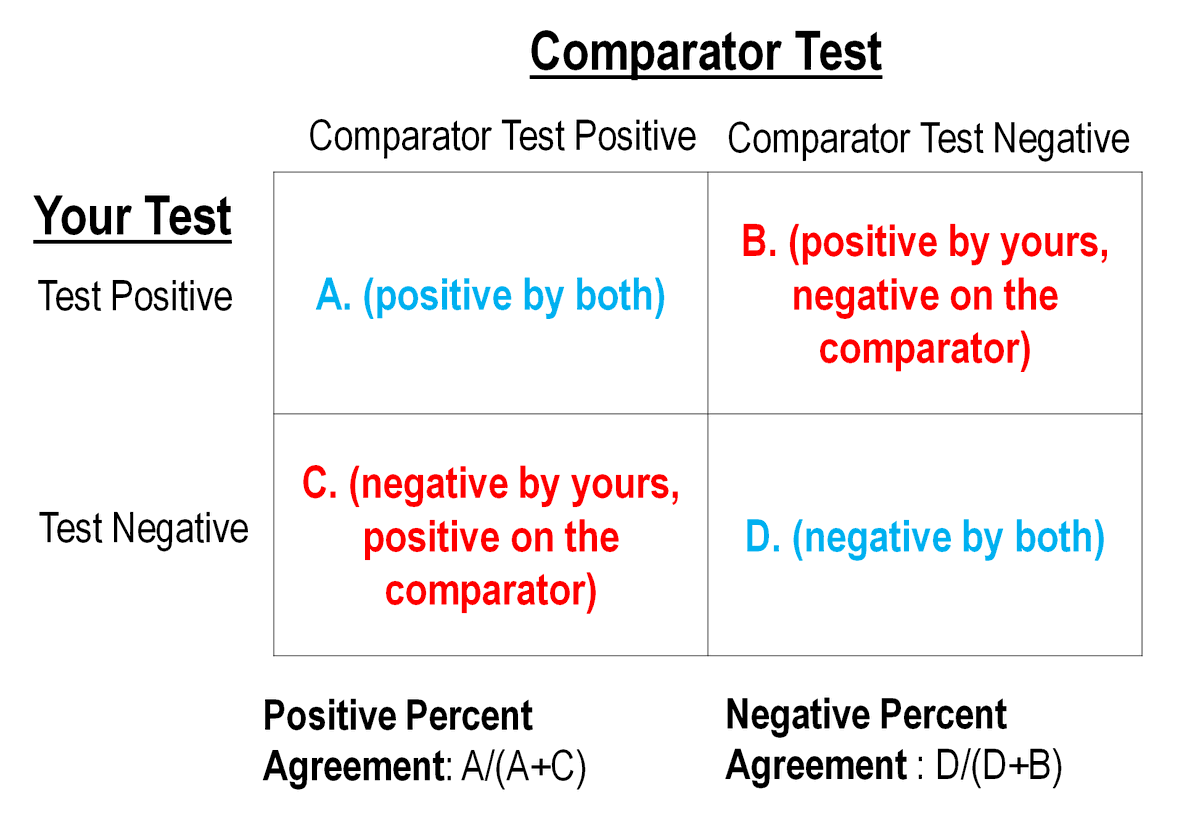
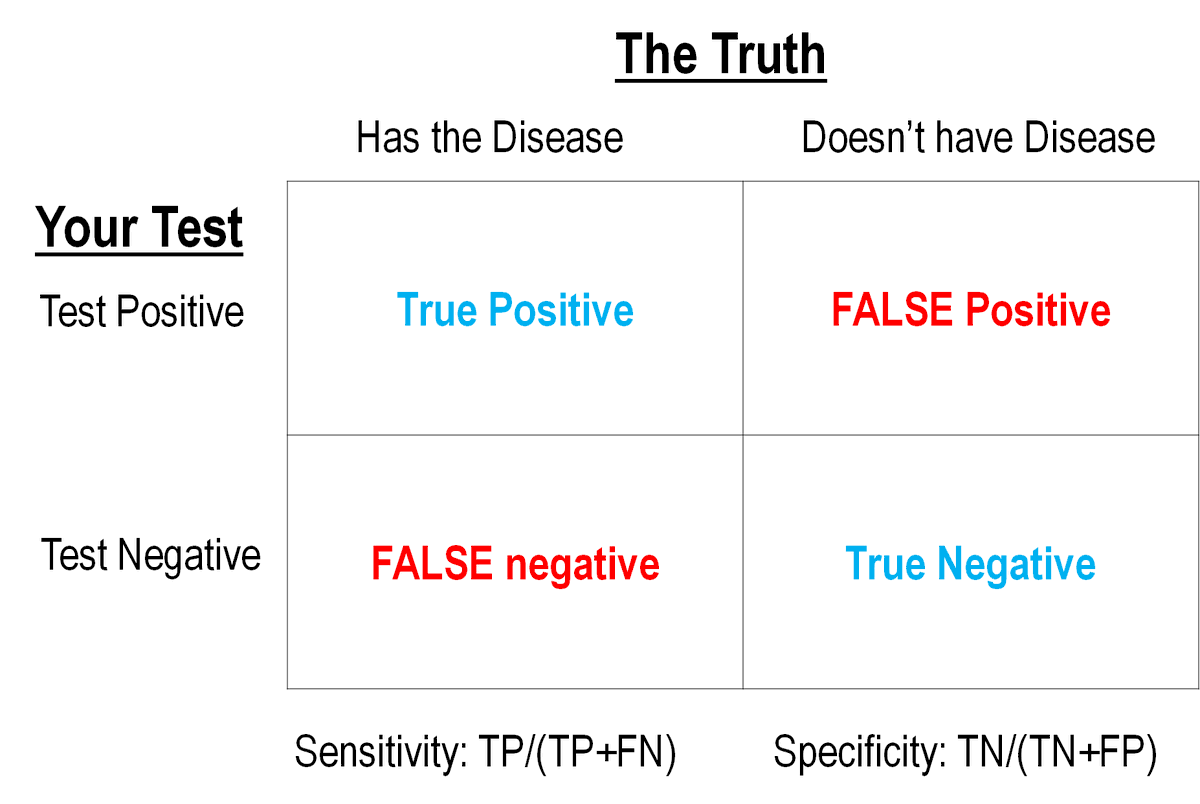
This can be calculated IF (and it's a crucial "if") you know the True Disease Status of people and then test them with your test approach. Like this:
2/
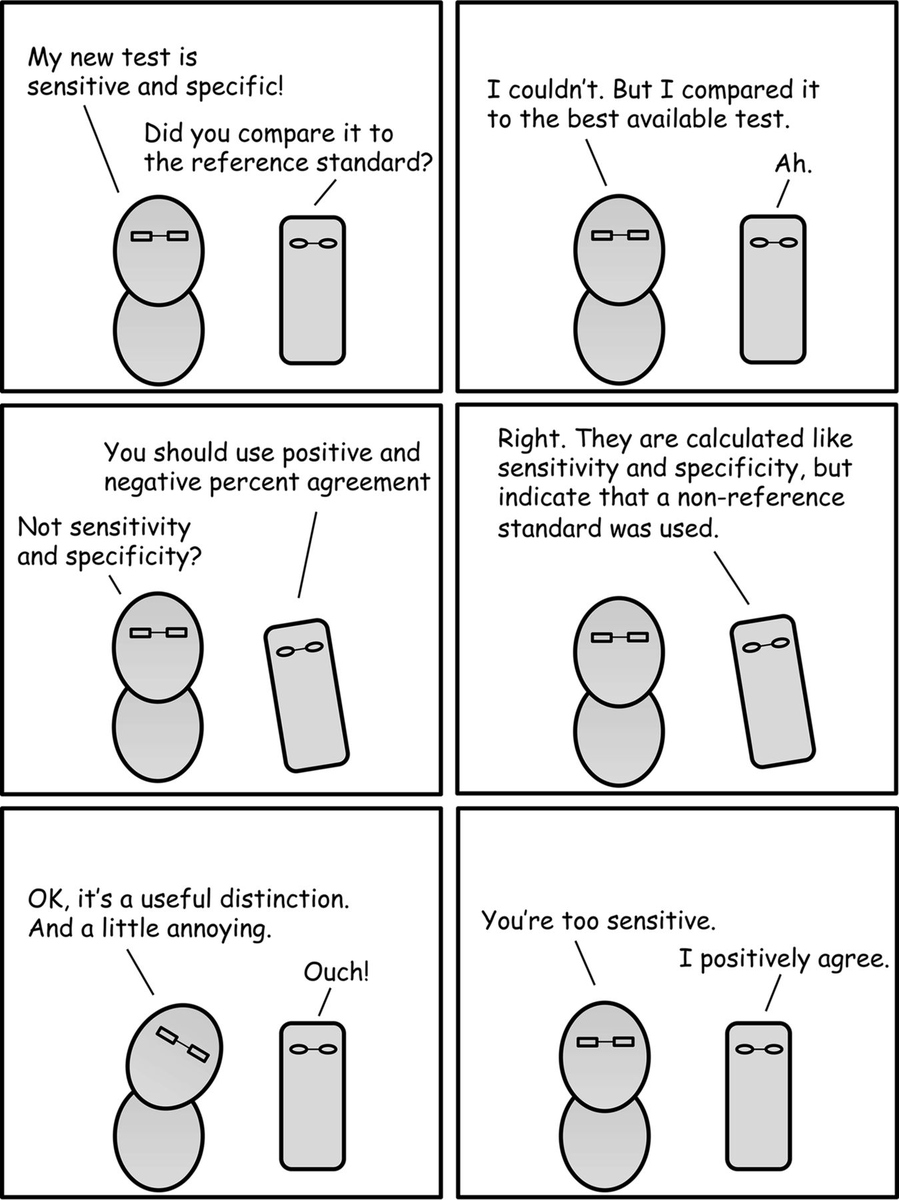
But it's more than pedantry to distinguish between them (see @JClinMicro's relevant comic): 4/
Relevant questions:
1. What samples were tested?
2. What was their comparator test?
3. What are the limitations?
5/n
fda.gov/medical-device…
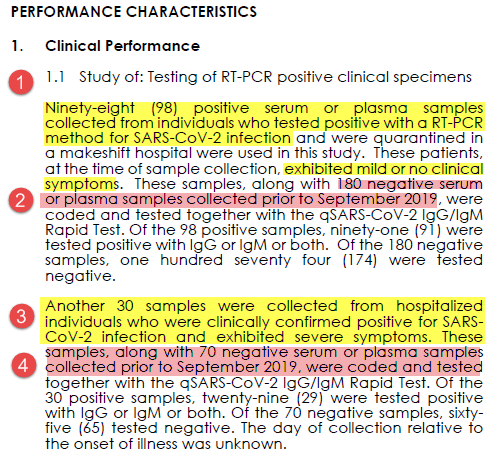
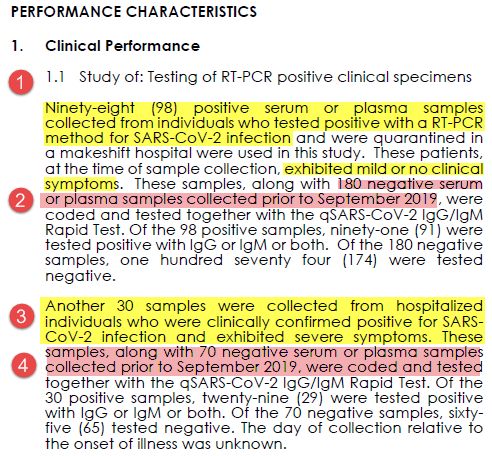
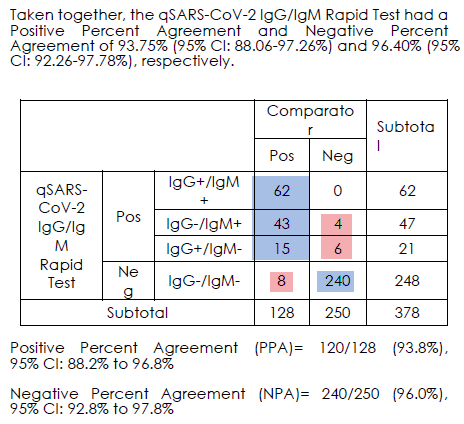
1. What samples tested? Serum from known PCR pos patients OR serum from before Sept 2019
2. What was the comparator test? PCR
Last is what might be the most important question:
3. What are the test limitations?
9/n
- When do antibodies arise in acute disease? (answer: too late to use it for acute diagnosis)
Here's a relevant pre-print - note sensitivity at d0-d7 medrxiv.org/content/10.110…
10/n
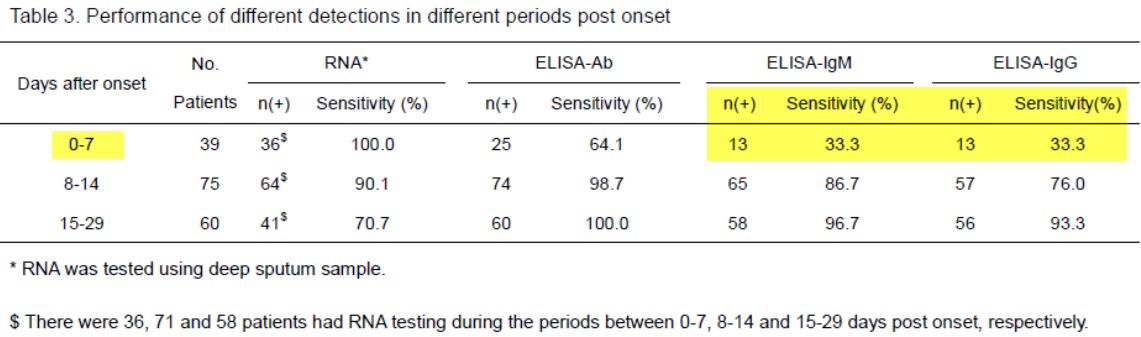
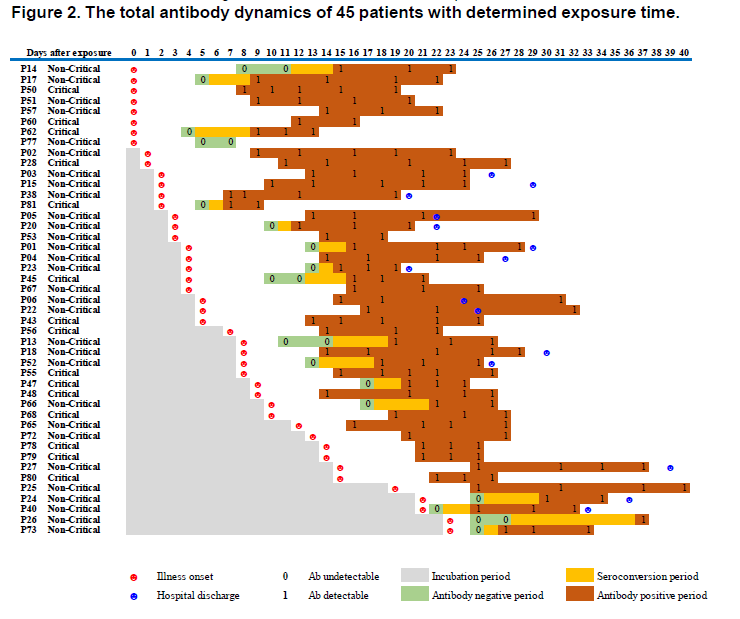
From the same preprint above ("Serology characteristics…" from Lou et al), this figure, normalized to date of exposure, showing illness onset.
Answer: it varies 11/n medrxiv.org/content/10.110…
-What kind of cross reactivity will there be seasonal Coronaviruses?
-Is anti-SARS-CoV2 IgG protective? (maybe? hopefully!) How long lasting is it?
etc.
Lots of questions remain is my point. 12/n
That's lots of words to say we REALLY need to understand test limitations! 13/n
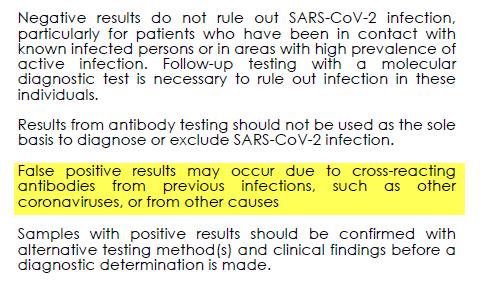
First, and my main focus here: We don't know the clinical sensitivity, specificity or predictive value of these tests. They aren't adequate for #COVID19 diagnosis & aren't equivalent to PCR tests.
14/n

But I don't see this type of rapid serological test filling either of those needs. 17/n
The End.