A quick thread on that topic, starting with @HelenBranswell's timely article (why aren't you following her??) as a jumping off point:
1/n
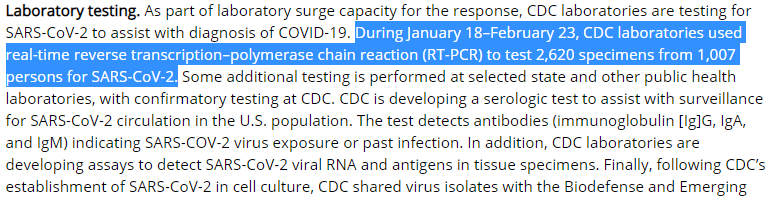
In an MMWR from last week, they said they'd tested "1,007 people" but news this week is saying "<500"? I don't understand that discrepancy.
Suffice to say it's far, FAR below what is needed! 2/n
But some component of that gave some labs problems (1 of 3 probes? a neg control? unclear to me)
cidrap.umn.edu/news-perspecti…
3/n

This means
1. criteria needs to be expanded!
2. testing needs to be available!
Both of these ASAP 4/n
statnews.com/2020/02/27/a-s…

If we know how the CDC's #COVID19 test works (& they openly published this a month ago) why can *any* lab copy that test and develop their own test?
cdc.gov/coronavirus/20… 5/n
Many good, valid clinical diagnostic tests are developed in-house by a laboratory (lab-developed test, LDT). LDTs are not (usually) reviewed by the FDA.
BUT... 6/n
EUA means FDA *does* review & authorize diagnostic tests for that disease. Currently: #COVID19 (previously: Zika, 2009-H1N1 etc)
For more see: 7/n
The EUA process is time-consuming, impractical and (importantly) NOT feasible for addressing the current need for #COVID19 testing 8/n
I'd say there's definite risk of fake or faulty "tests" made and sold by unethical people trying to capitalize on public fear.
Solution: streamline authorization for trusted diagnostic labs & groups! 9/n
We're FAR below the capacity of what testing COULD be happening w/ faster, better collaboration. 11/n
Follow @UNC_Clin_Micro's example: encourage your elected officials to help streamline the FDA's EUA system and to support public & diagnostic labs!
We're all in this together! 12/12
Along w/ travel & contacts, that's now part of the criteria too.
13/12
The first test isn't the only test (or the best one?) 14/12
I think we really need more details here, but it sounds like the FDA is moving toward exactly what was needed: letting labs develop their own #COVID19 tests!
Clinical diagnostic labs, some with #COVID19 tests ready and waiting, were not allowed to do so due to FDA guidelines restricting diagnostic tests in emergency (EUA) settings.
What @ddiamond reports is: that may change! Hurray!











