#SARSCoV2 #COVID19 got you down? Me too.
Ready for some good news? Here it is: #Dexamethasone (dex) works.
But when, how much, and for which patients?
Here’s #HowIReadThisPaper on Horby et al: the RECOVERY trial prelim report: nejm.org/doi/full/10.10…
(Thread)

Background: COVID19 can induce a deadly hyper-inflammatory host response.
Prior observational data (↓quality, ↑risk of confounding by indication) suggested ↑mortality from steroids in influenza: pubmed.ncbi.nlm.nih.gov/30798570/
The role of steroids in treating COVID19 is unknown.
Question: What is the effect of dexamethasone on all-cause mortality among patients hospitalized with COVID19?
Date published:
- press release: 16 June
- preprint: 22 June
- manuscript: 17 July 2020
Funding: National Institute for Health Research (NIHR), UK & others
Study design: multicenter, open-label (unblinded), pragmatic randomized clinical trial
RECOVERY (recoverytrial.net) is an ongoing platform trial aiming to randomize 15,000 patients through Dec 2031 to one of 7 treatment arms (!).
These are the results of the dex arm.
3 RECOVERY treatment arms are now closed:
Lopinavir-ritonavir (futility)
Hydroxychloroquine (futility)
Dexamethasone (success!)
Ongoing arms:
Azithromycin
Convalescent plasma
Tocilizumab
No trial intervention (usual care)
From here on, we’ll focus on dex vs usual care.
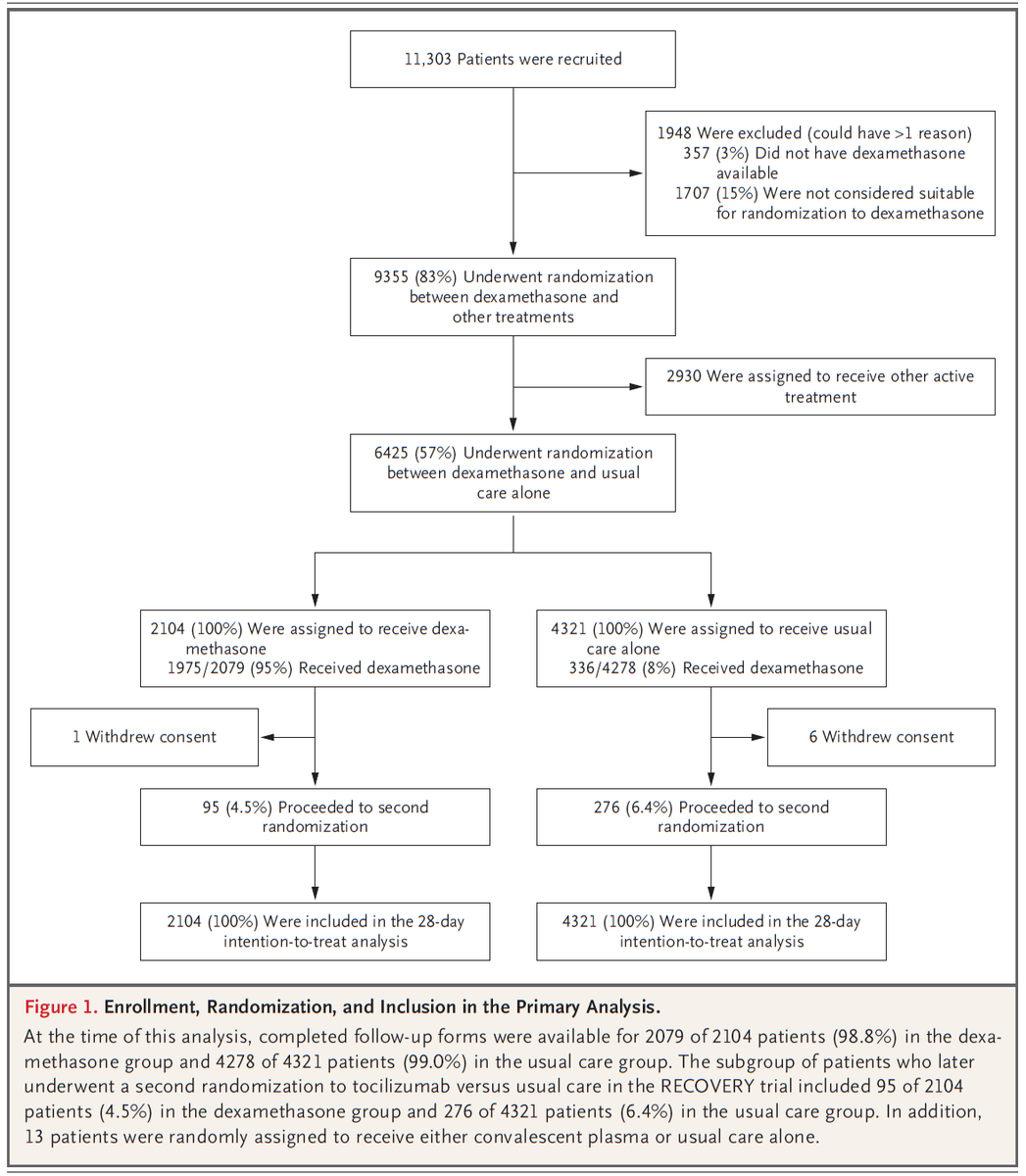
Population: 6,425 patients hospitalized at one of 176 NHS hospitals in the UK with clinically suspected or confirmed COVID19.
2,104 were randomized to dexamethasone, and 4,321 to usual care.
Study period: 3/19/2020 - 7/6/2020
Inclusion Criteria: hospitalization for suspected or confirmed COVID19
Exclusion Criteria: attending physician believed there was a contraindication OR an absolute indication for dexamethasone, or dexamethasone was unavailable at the time of enrollment
Study Procedures: A single online follow-up form, completed 28 days after randomization (or on date of discharge or death), collected info on:
-adherence
-other treatments
-LOS
-respiratory & renal support
-vital status (including cause of death)
Not recorded: adverse events
Protocol available? Yes: recoverytrial.net/files/recovery…
and
clinicaltrials.gov/ct2/show/recor…
Primary outcome: All-cause mortality at 28 days
Secondary outcomes: live discharge within 28d, clinical worsening (requirement of intubation or death)
Primary analysis: Intention-to-treat survival analysis
Sample size justification: Assuming 28-day mortality of 20% in control group, 2,000 patients in dex group + 4,000 in control group = 90% power to detect 4% absolute (or 20% relative) reduction with two-sided alpha=0.01.
Authors’ conclusions: Dex 6 mg QD for <10d, added to usual care for patients hospitalized with COVID19, was associated with a 4.1% absolute ↓ in 28-day all-cause mortality among patients requiring O2, and a 12.3% ↓ among patients who were intubated & had symptoms for >7d.