Should we use #Lopinavir-ritonavir (LPV/r) + #Ribavirin (RBV) +/- #Interferon beta-1b (IFN) to treat #SARSCoV2 #COVID19? Let’s take a look at the data.
Here’s: #HowIReadThisPaper on @TheLancet trial of triple therapy for COVID19
Hung et al: thelancet.com/journals/lance…

First, let’s assess our baseline beliefs about triple therapy:
Before reading this study, when considering triple therapy as a treatment for patients with COVID19, I think the most important component is likely to be:
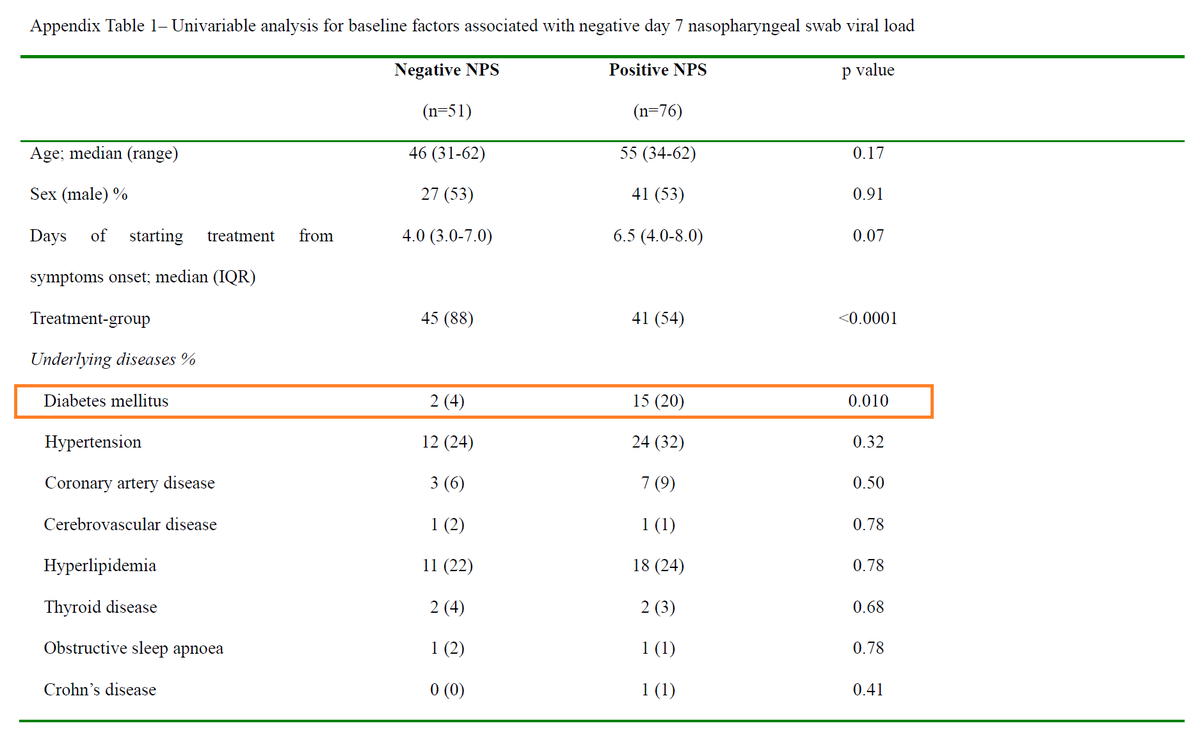
Background: A 2003 case series suggested ↓mortality (vs historical controls) in SARS patients treated with LPV/r + RBV :
ncbi.nlm.nih.gov/pmc/articles/P…
In 2015, LPV/r and IFN led to ↓viral load and improved clinical outcomes in animal models of MERS:
pubmed.ncbi.nlm.nih.gov/26198719/
Question: Does the triple combo of LPV/r + RBV +/- IFN reduce viral load and improve clinical outcomes in patients hospitalized with COVID19?
Date Published: 8 May 2020
Funding: Foundations + individuals; no drug manufacturer support
Study Design: Multicenter, randomized, open-label (unblinded) active-controlled (no placebo group) superiority trial
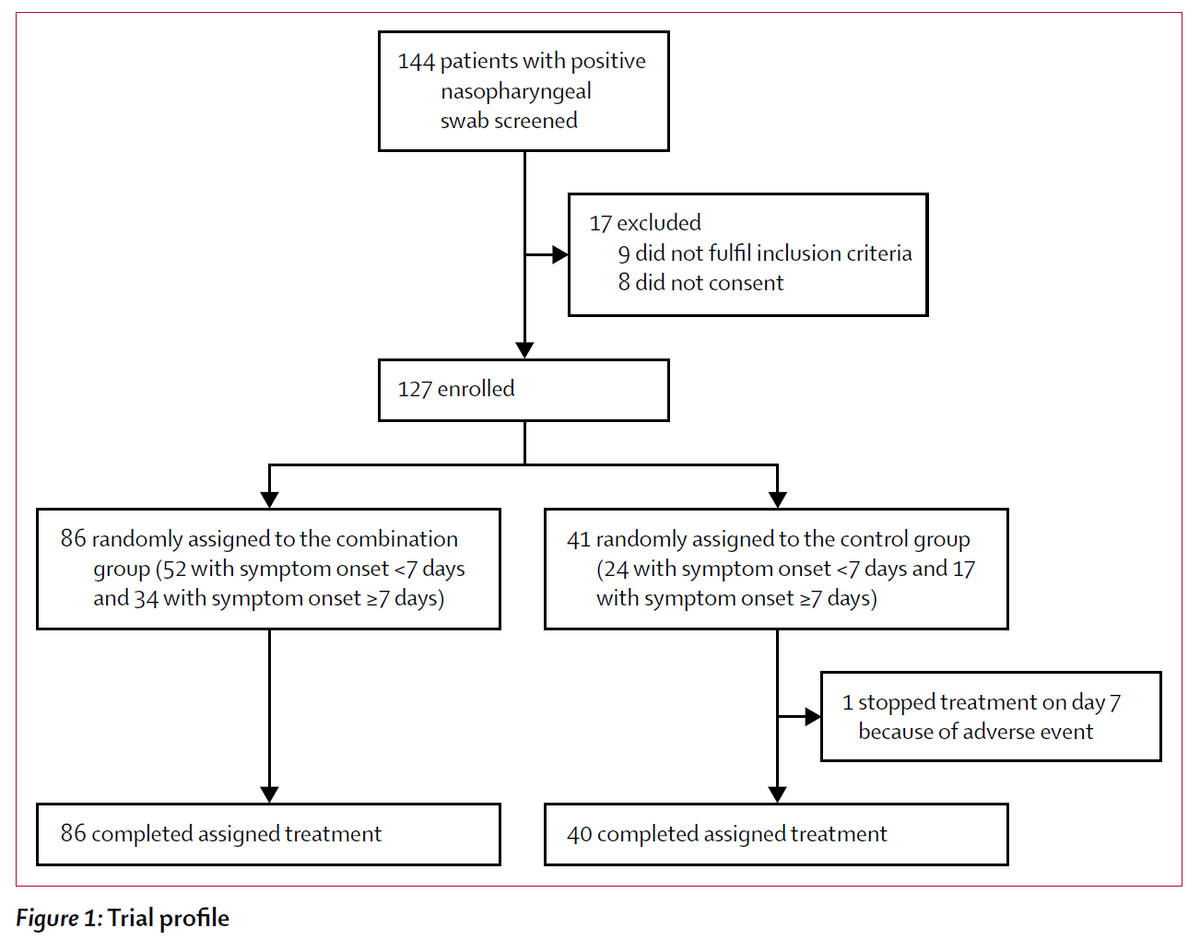
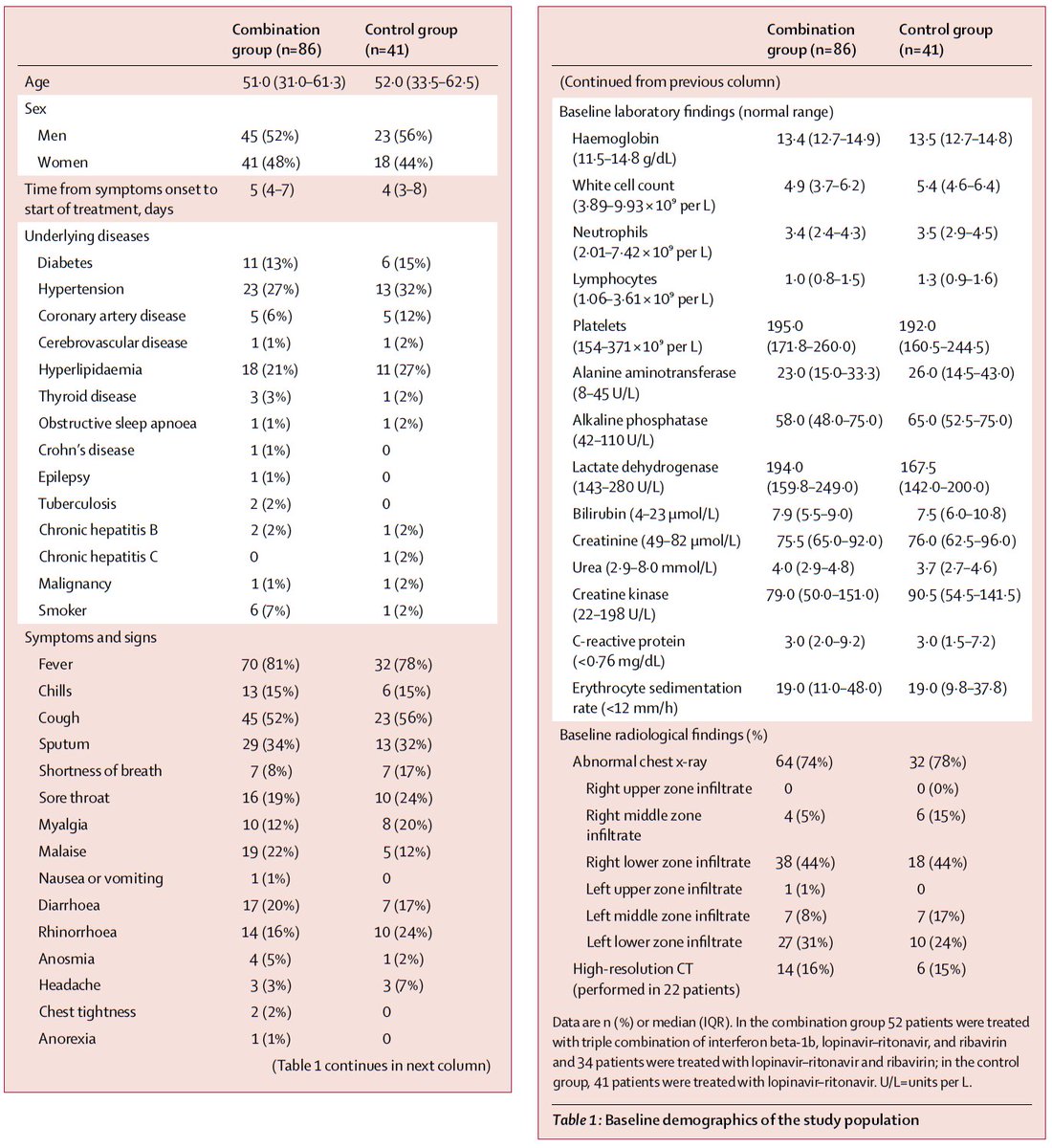
Population: 127 adults hospitalized with PCR-confirmed COVID19 at 6 hospitals in Hong Kong were included
Study Period: 2/10/2020 - 3/20/2020
Intervention: 14d of:
LPV/r (PO)
+
RBV (PO)
+/-
IFN (SubQ)
3 doses if 1-2d of Sx; 2 doses if 3-4d of Sx, 1 dose if 5-6d of Sx, 0 doses if >6d of Sx
+
Usual care
Control: LPV/r x14d + usual care
Usual care = any level of O2, dialysis, steroids, or ABx as indicated
Study Procedures: Consecutively admitted patients were enrolled within 48h and randomized 2:1 (triple therapy : control) without stratification.
All patients requiring O2 received stress-dose steroids.
Daily swabs for PCR from multiple sites were collected until discharge.
Protocol available? Yes: clinicaltrials.gov/ct2/show/recor…
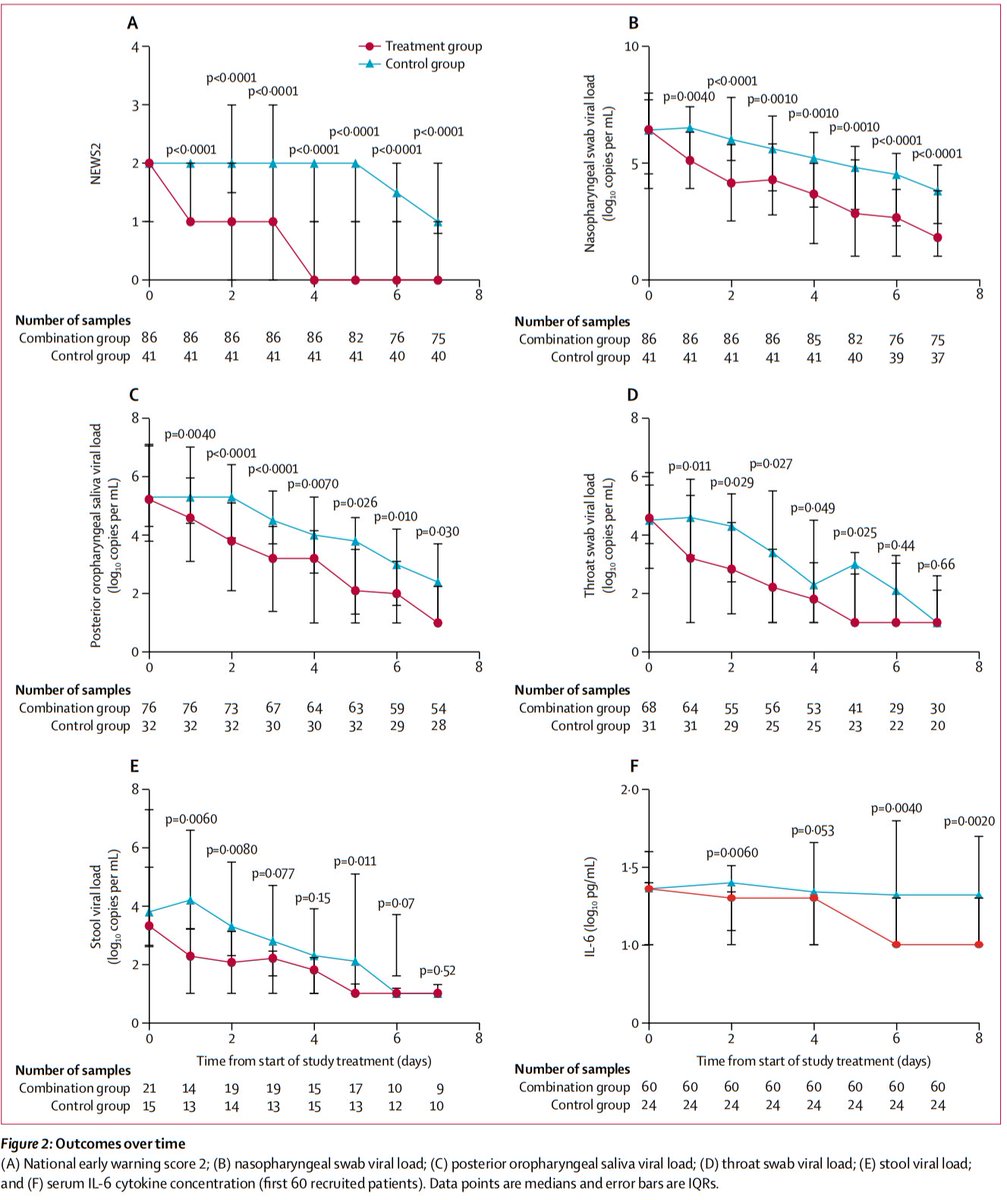
Primary outcome: Time to negative nasopharyngeal swab PCR
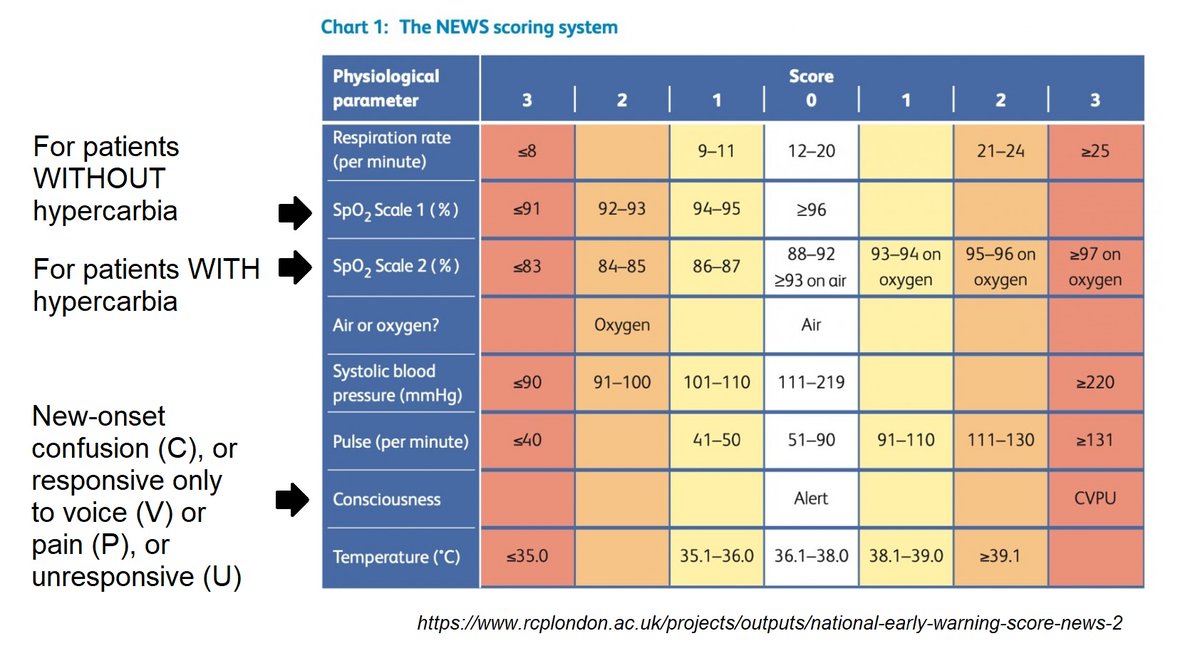
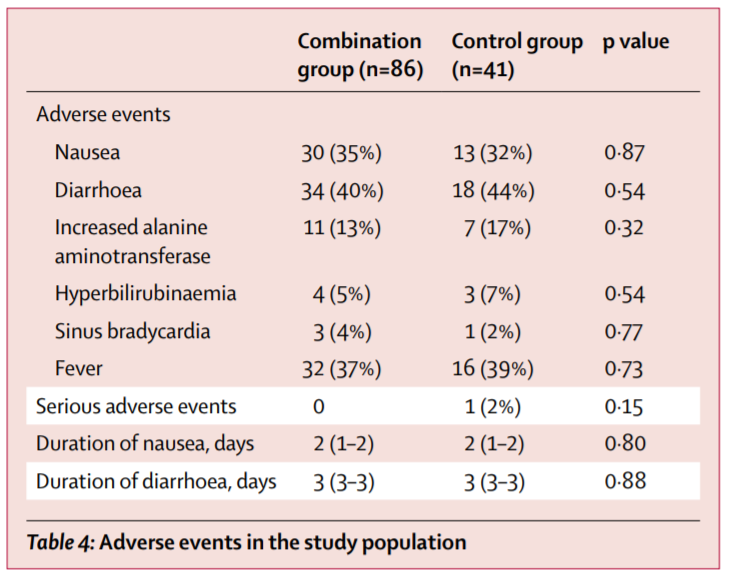
Secondary outcomes: Resolution of Sx (defined as NEWS2=0 for >24h), LOS, 30-day mortality, detection of mutations, adverse events
Primary analysis: Time-to-event, intention-to-treat population
Sample size justification: 70 patients = 80% power (w/ 2-sided alpha 0.05) to detect a 26% absolute reduction in 21-day mortality or ARDS (allowing 17% dropout) assuming event rate of 29% in the control group
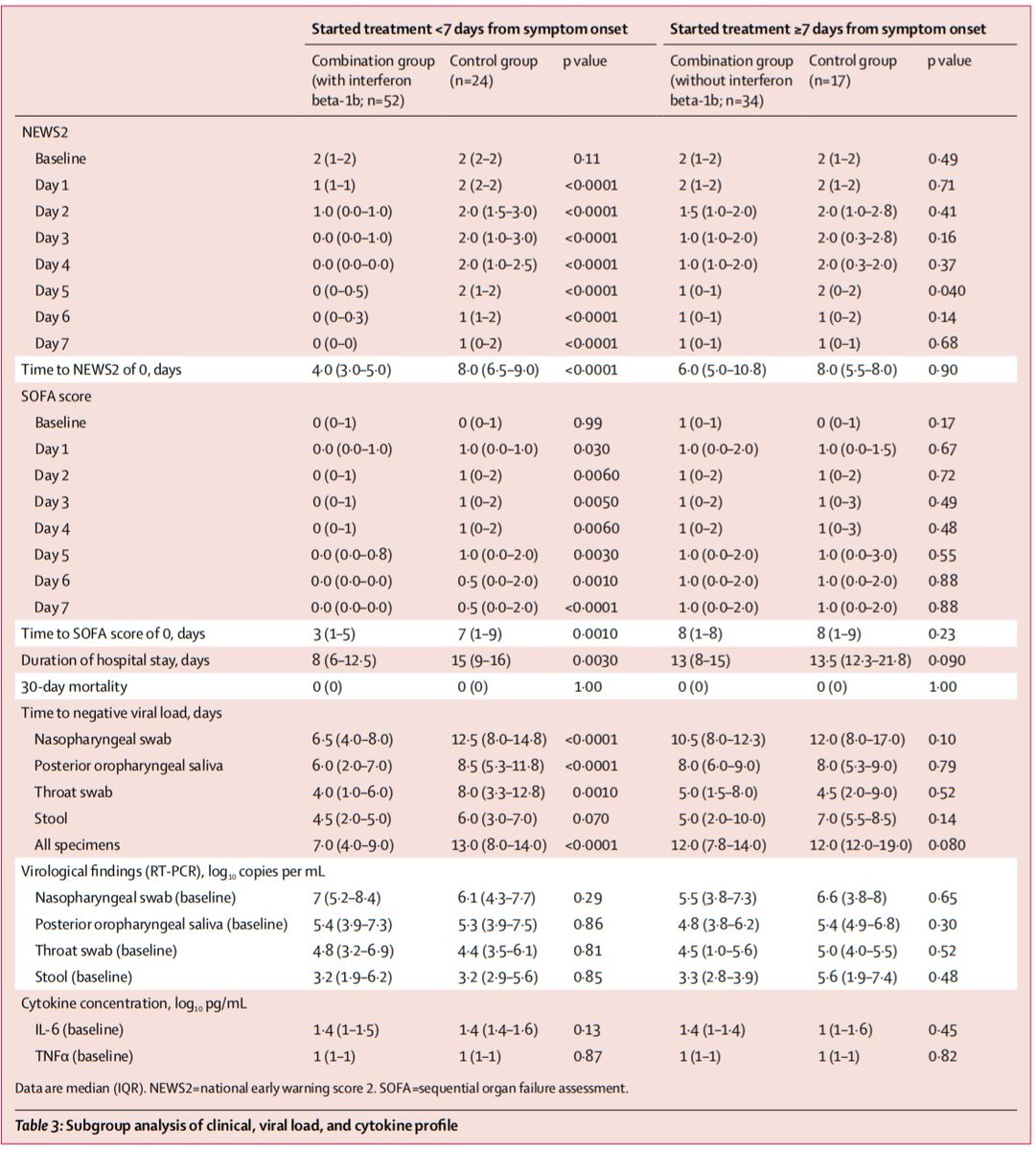
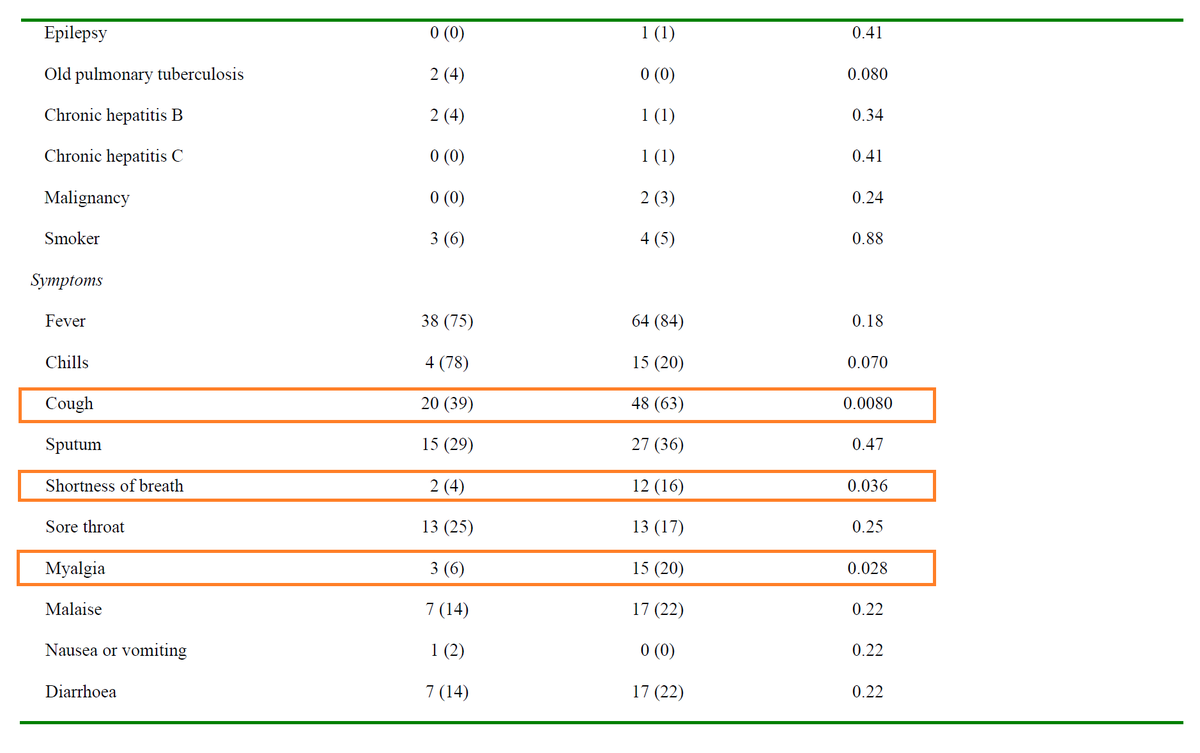
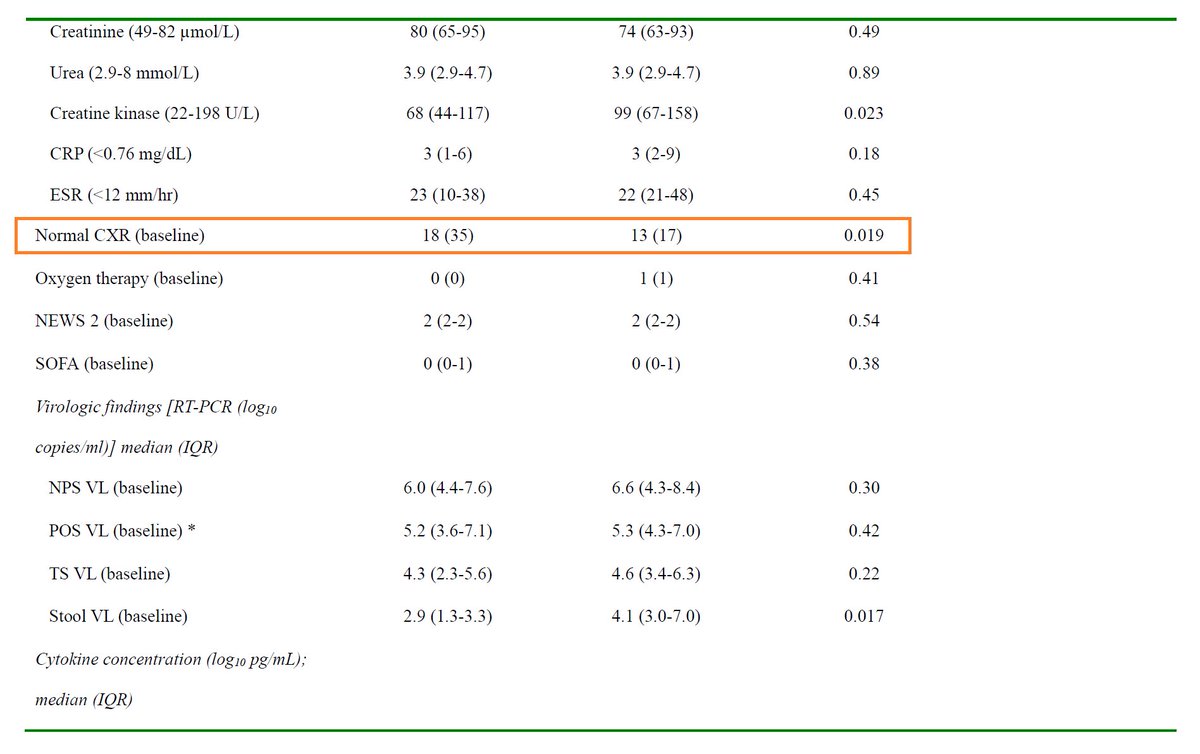
Secondary outcomes (triple therapy vs control):
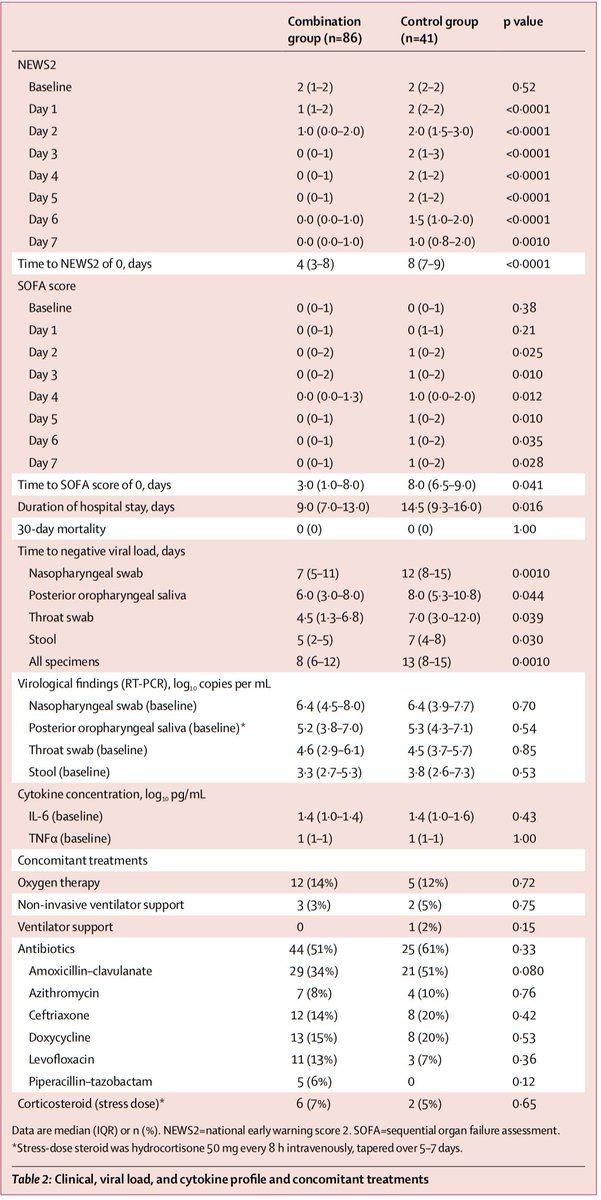
Time to NEWS2=0: 4 vs 8 days (p<0.0001)
LOS: 9 vs 14.5 days (p=0.016)
30-day mortality: 0 vs 0
No mutations in the nsp5 gene (which antivirals could select for) were detected.
Author’s conclusions: Added to usual care, triple therapy with LPV/r + RBV +/- IFN was associated with a 5 day reduction in time to negative NP swab, compared with LPV/r alone. This effect was driven by patients receiving IFN (those with Tx <7d of Sx onset).
My appraisal: This was a positive study.
Does this mean we should use triple therapy to treat patients hospitalized with COVID19, or could chance or bias explain these findings?
Let’s take a deeper look.
It should be noted that the primary outcome in this study (time to negative NP swab) is a surrogate outcome.
What we are really interested in is whether triple therapy reduces morbidity and mortality from COVID19.
Does this study provide any clues?
Also, the study population was not very sick (only <15% on O2; no deaths). This means a very large sample size would have been needed to show any mortality difference, if one exists, because deaths were rare.
Why was this the case? The authors note that a public health ordinance in Hong Kong required ALL patients with RT-PCR confirmed SARSCoV2 to be hospitalized.
This limits the generalizability of these results to settings where hospitalized patients are much sicker.
It did appear that triple therapy was associated with faster resolution of vital sign abnormalities (as reflected by NEWS2=0), but info on resolution of actual symptoms was not provided.
Did triple therapy prevent worsening? We don’t know, but it is a testable hypothesis.
Also, a negative NP swab x2 was a criterion for discharge, so the ↓LOS could be reflective of reduced time to a negative swab rather than an impact on disease severity.
So, from this study, we can’t say if triple therapy reduces morbidity & mortality.
However, though PCR positivity is not a surrogate for shedding of infectious virus, a negative PCR does reduce the post-test probability of viral shedding, and therefore, contagiousness.
This suggests these findings, if true, could have implications for ↓transmission.
Another caveat is that the duration of PCR positivity in the control group was much shorter than in other studies.
In this case series, viral shedding was detected up to 4-6 weeks after symptom onset:
academic.oup.com/cid/advance-ar…
To close, let’s revisit the original question:
After reading & appraising this study, when considering triple therapy as a treatment for patients with COVID19, I think the most important component is likely to be:
Bottom line: Among patients hospitalized with mild disease from COVID19, triple therapy was associated with reduced time to a negative NP swab; this was driven by IFN and/or Tx <7d of Sx onset. Further study is needed using clinical endpoints in sicker patients.
(End)