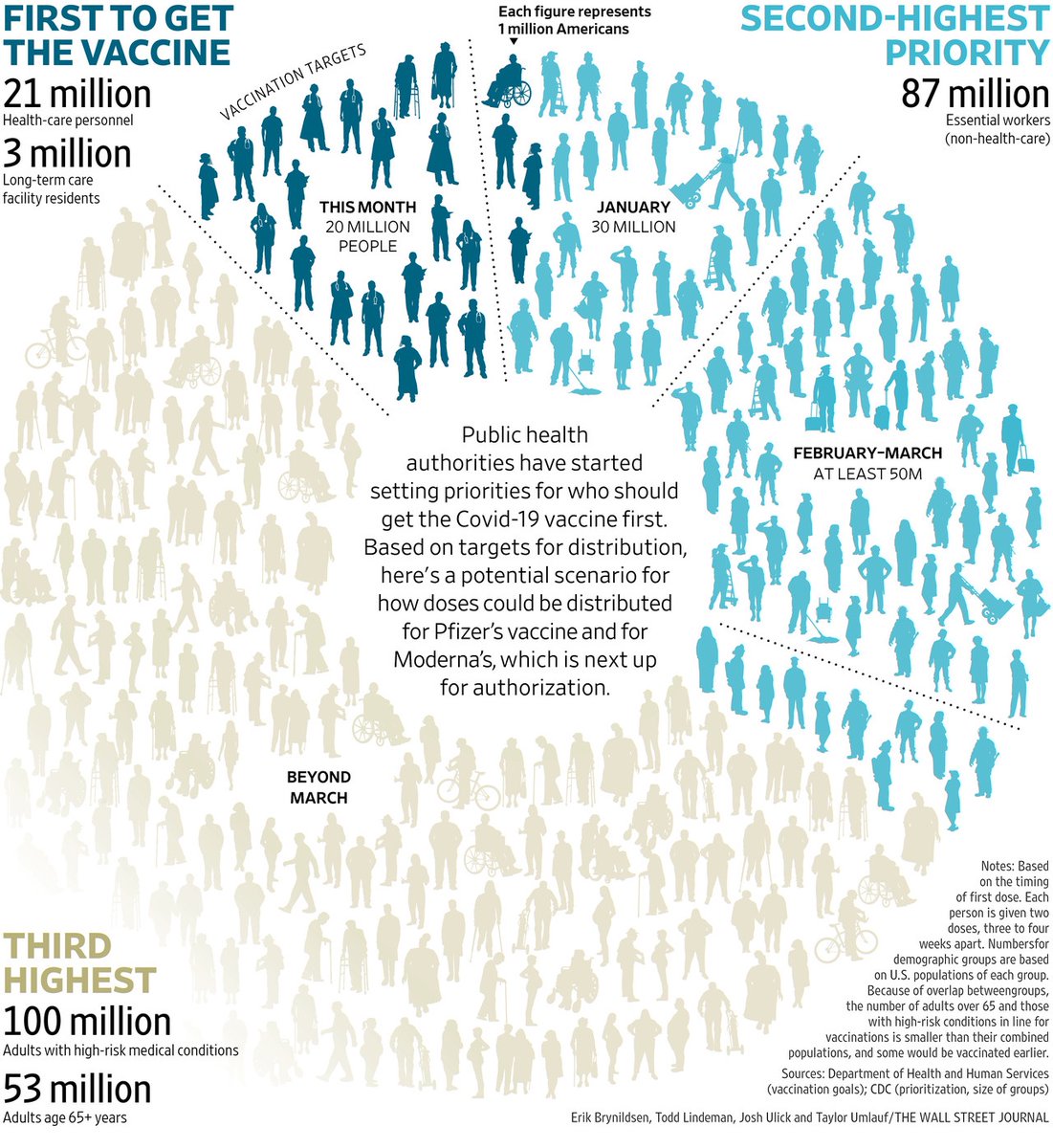
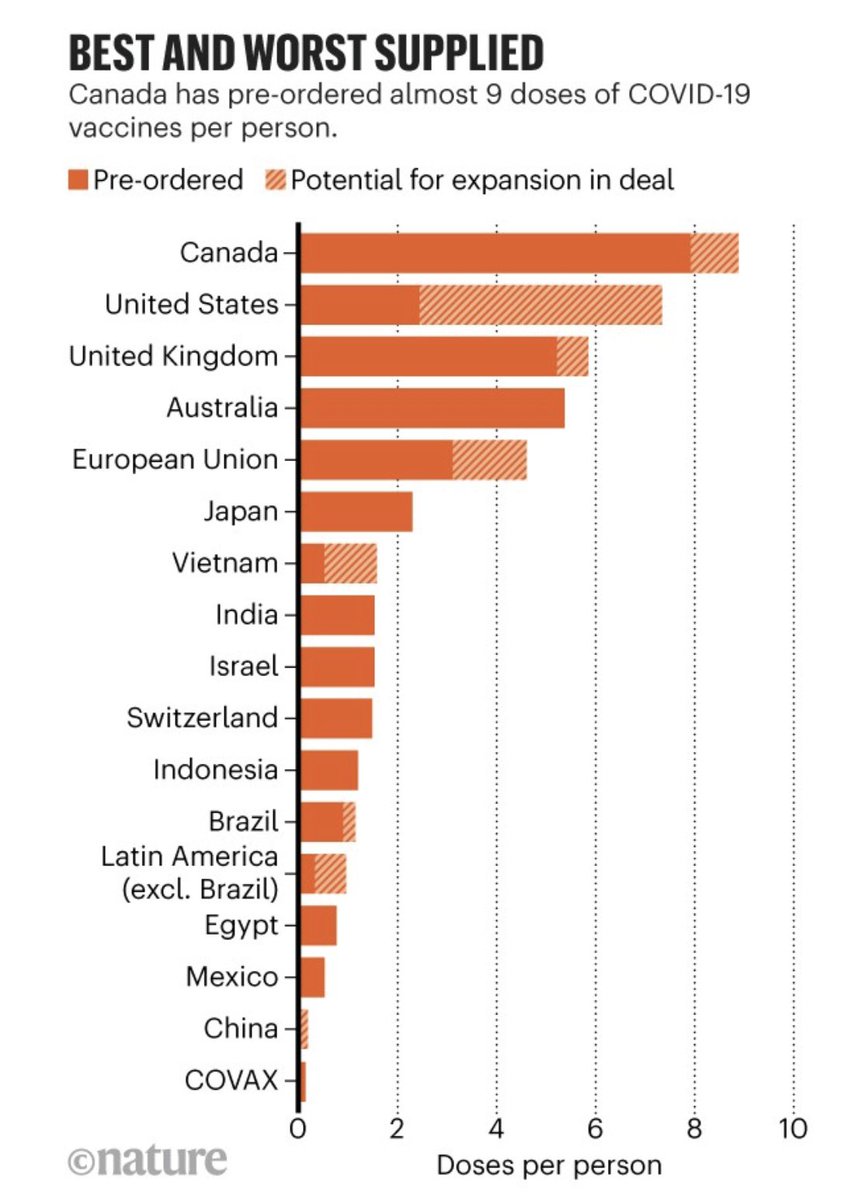
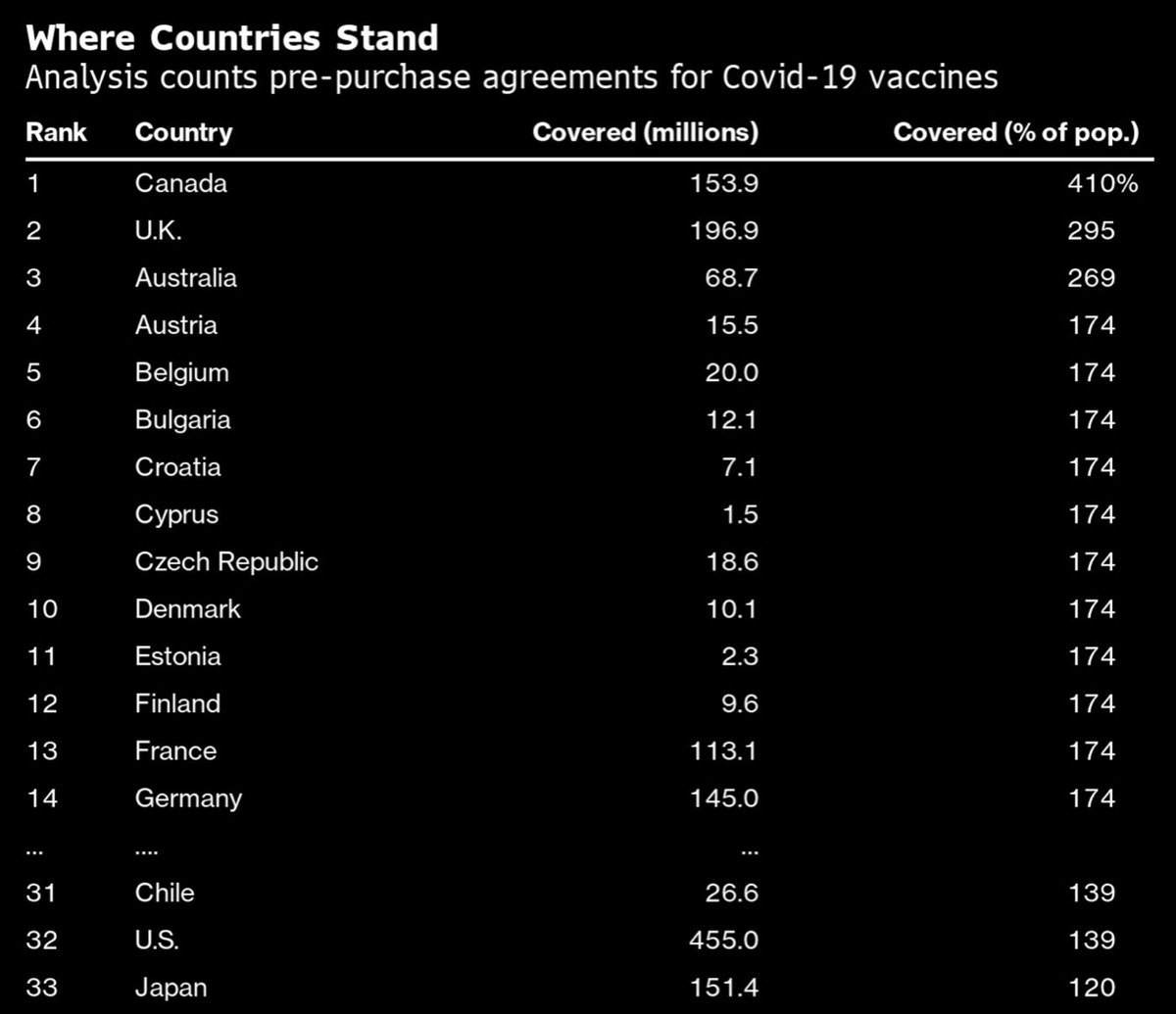
How do you get to over 240,000 covid cases in one day?
Ask the USA.
An unabated virus.
A rudderless country.
Today @COVID19Tracking
Ask the USA.
An unabated virus.
A rudderless country.
Today @COVID19Tracking
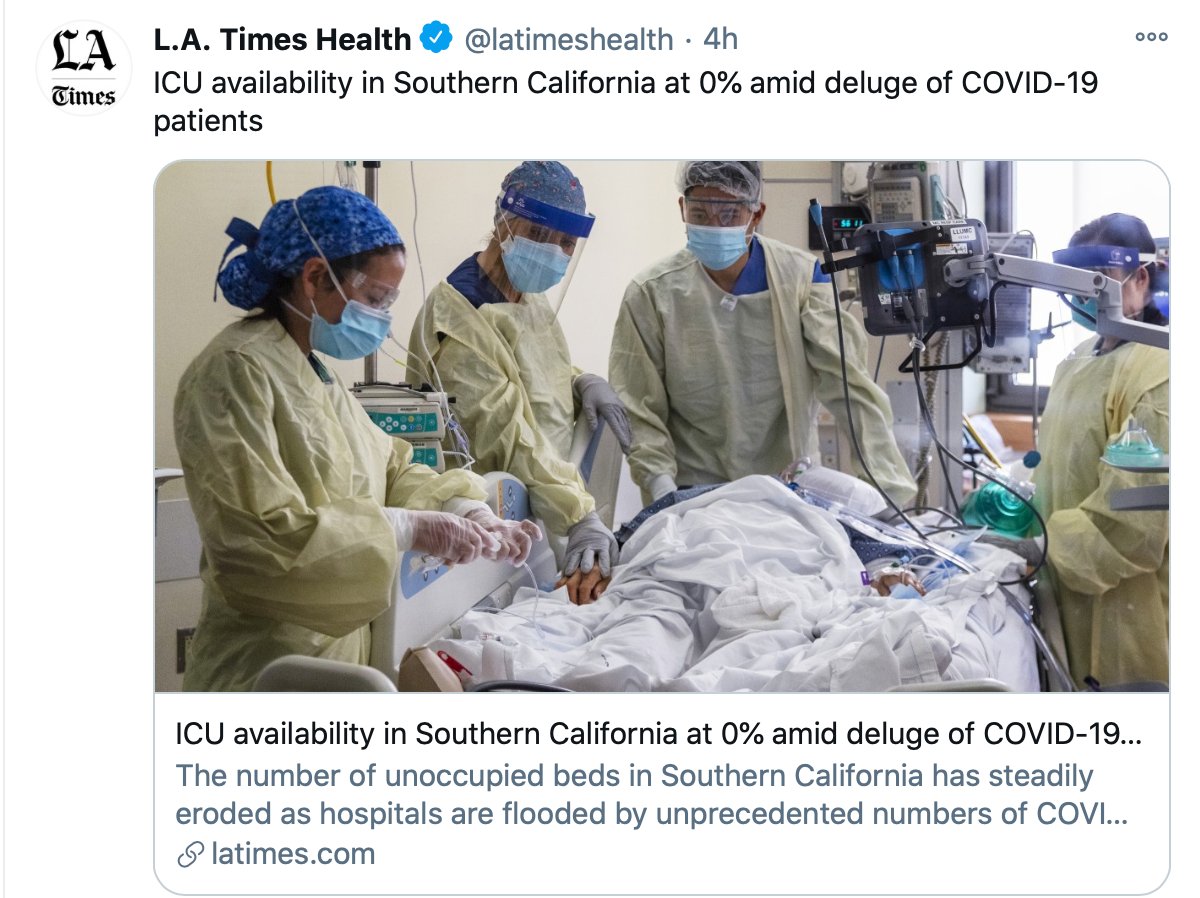
Our status in San Diego and LA.
Hope yours is better

Hope yours is better
https://twitter.com/latimeshealth/status/1339686066253524993
• • •
Missing some Tweet in this thread? You can try to
force a refresh