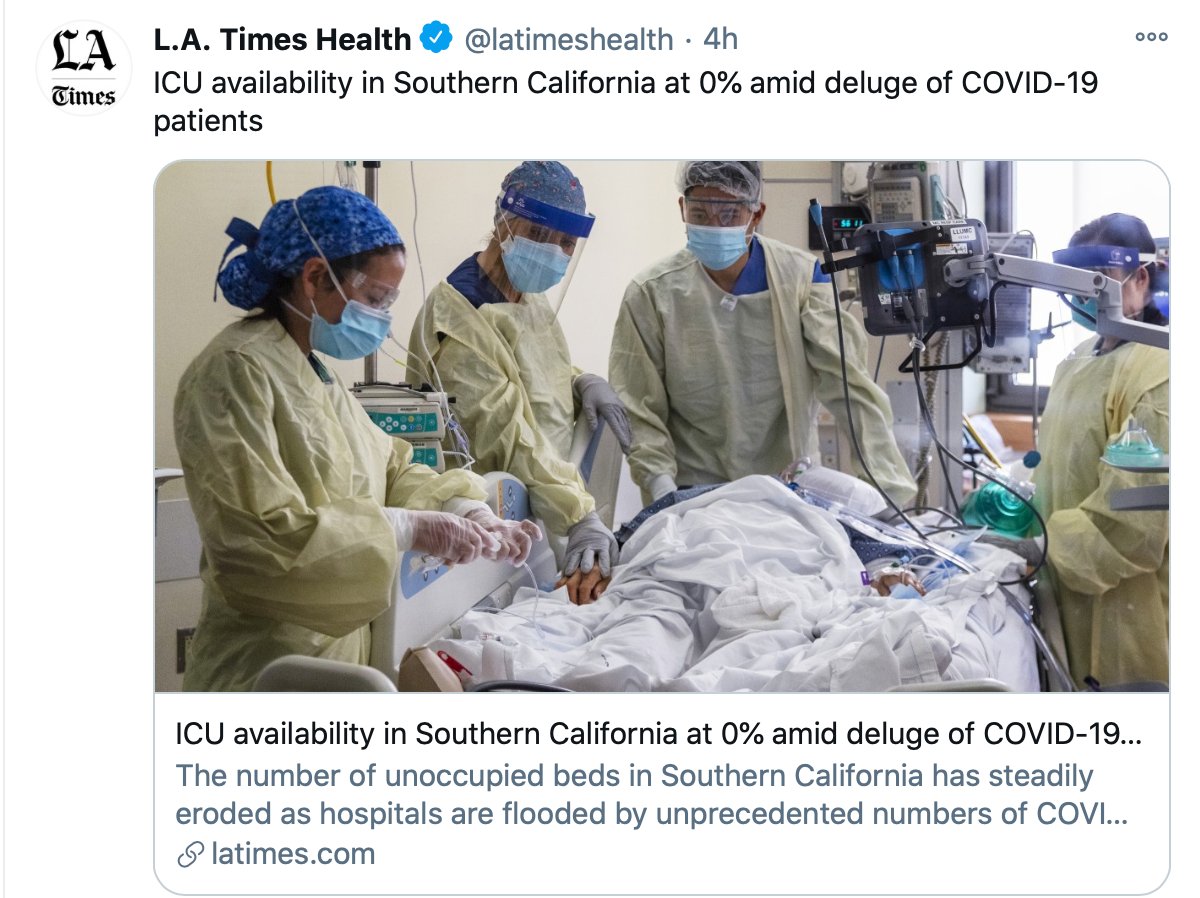
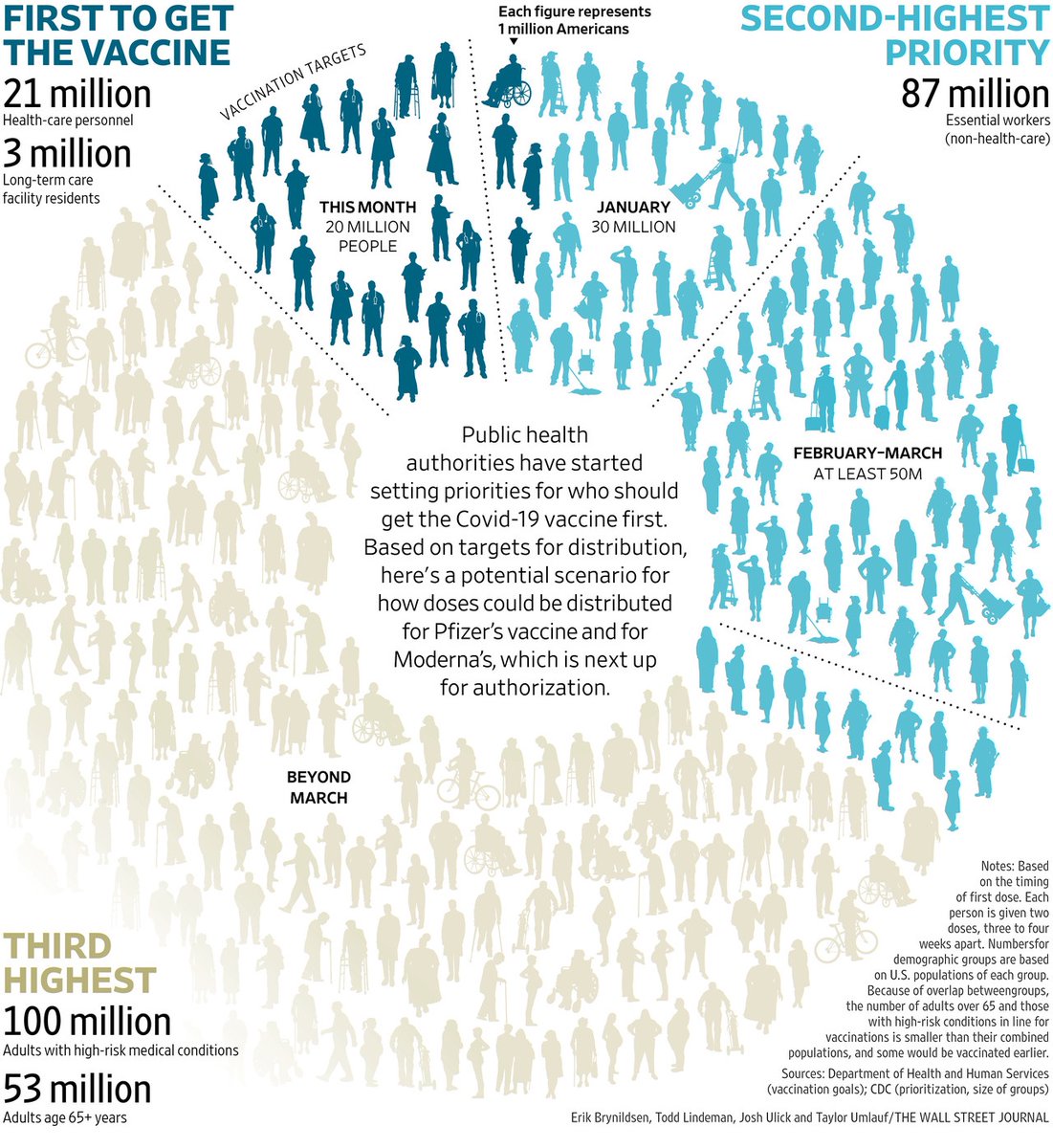
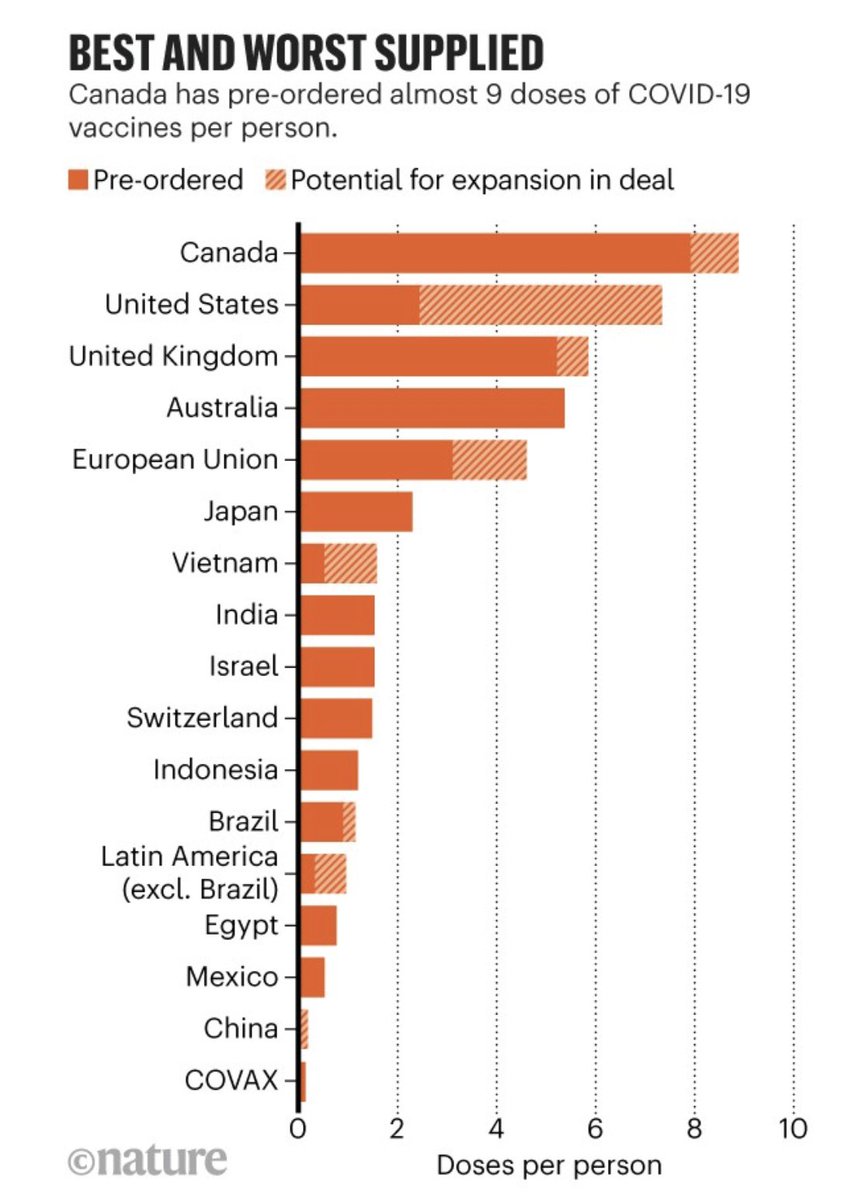
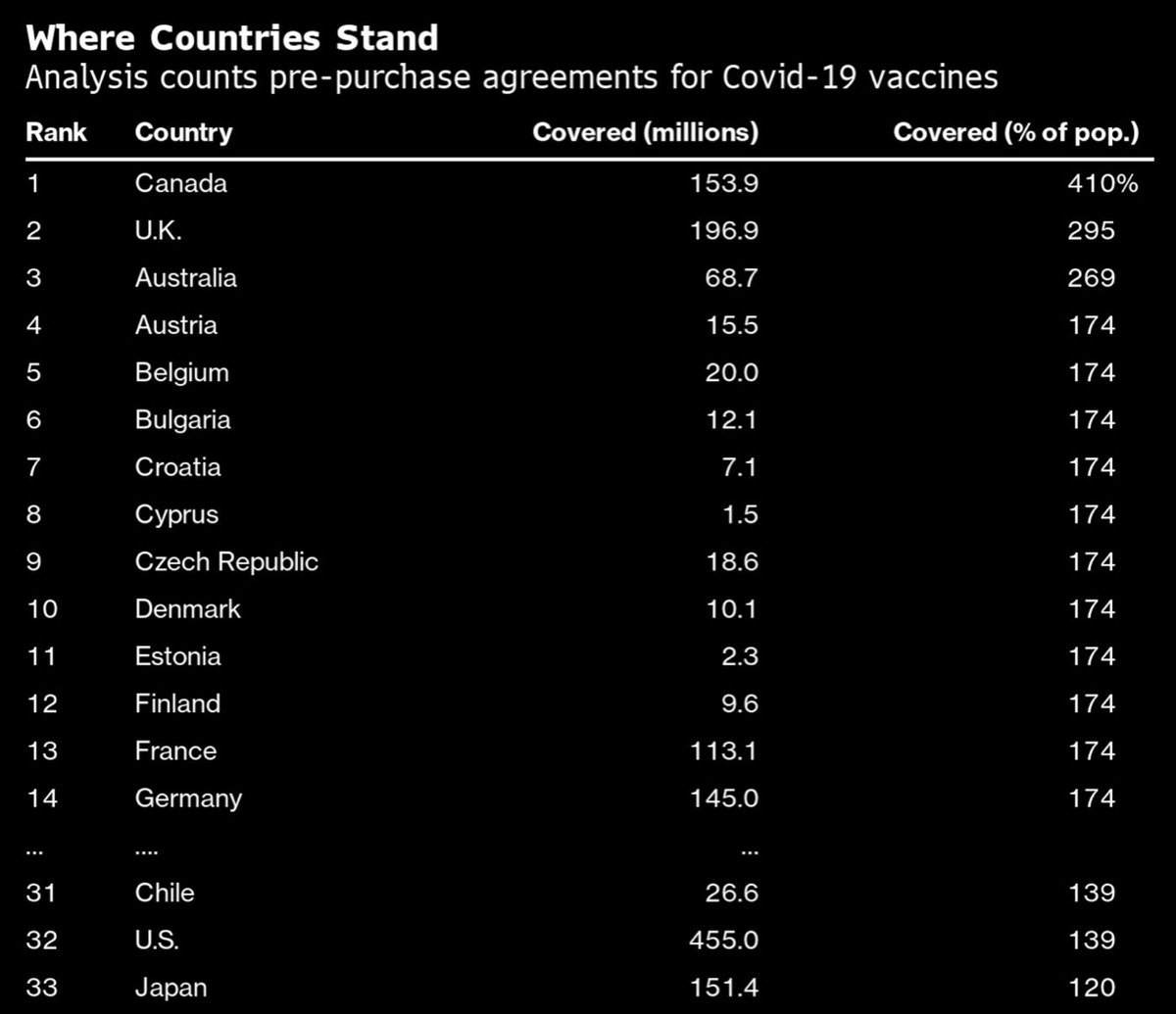
1. There are limited data about the vaccines for people who have had covid. But we can learn from the @Pfizer @BioNTech and @moderna trials which had 3% and 2.2% participants respectively confirmed at baseline (via FDA briefing docs)
2. In the Pfizer trial there were 9 reinfections among 670 participants (1.3%) who received placebo which was the same rate as those without prior infection (259 of 19,818 participants, 1.3%).
Only 1 reinfection in the vaccine group (after ≥ 7 days) for those w/ prior infection
Only 1 reinfection in the vaccine group (after ≥ 7 days) for those w/ prior infection
3. We need to learn more about this with so many people with prior infections getting vaccinated. But this finding suggests vaccine-induced immune response may add protection to the natural response and adds another layer to the superhuman concept 👇
https://twitter.com/EricTopol/status/1333426148571709447
4. Here is the link fda.gov/media/144245/d…
and the text, data
@moderna_tx didn't provide the details of their 2.2% of prior infected participants
and the text, data
@moderna_tx didn't provide the details of their 2.2% of prior infected participants

• • •
Missing some Tweet in this thread? You can try to
force a refresh