#sequentScientific #Q3marketupdates #Q3investorpresentations
Strong 🏋️♀️
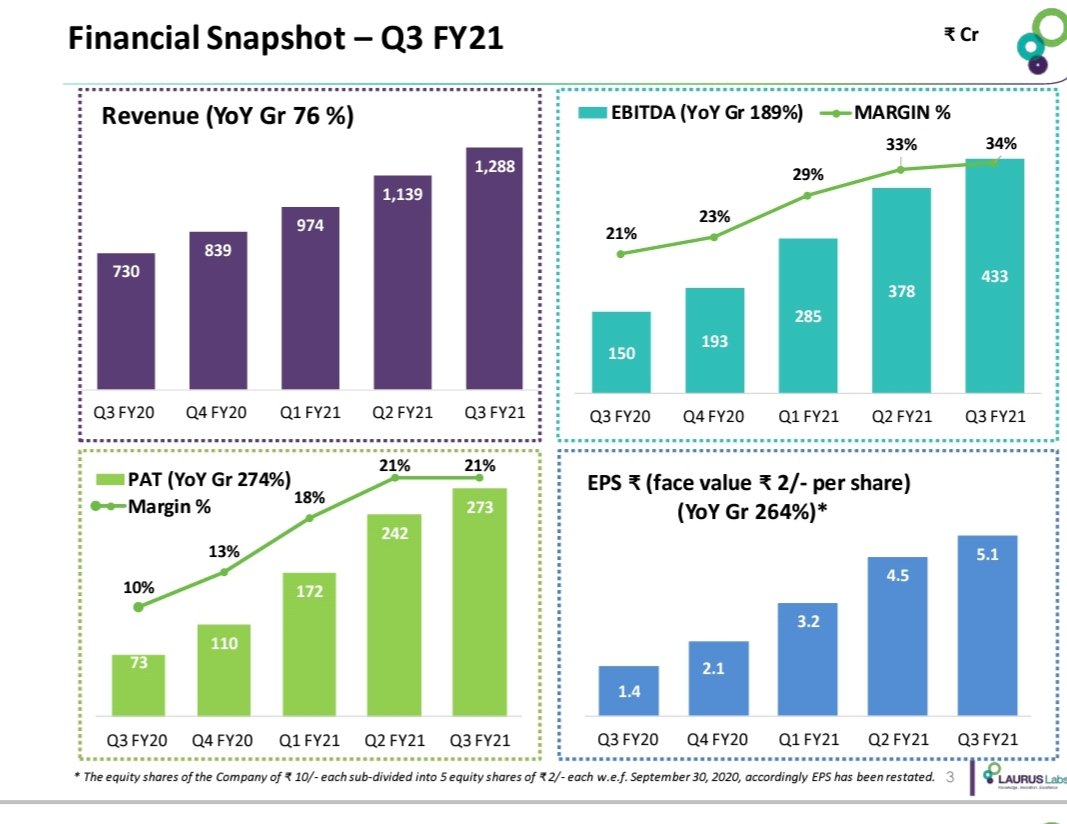
Q3fy21/20 in cr
Rev 361/317
PAT 37.2/20
EPS 1.52/0.83
9mnth fy21/20
Rev 1014/878
PAT 87.8 /53.1
EPS 3.24/2.18
API Business up 17.2%
Formulations 15.0%
Alivira award best Company in AH India/ME/Africa


Strong 🏋️♀️
Q3fy21/20 in cr
Rev 361/317
PAT 37.2/20
EPS 1.52/0.83
9mnth fy21/20
Rev 1014/878
PAT 87.8 /53.1
EPS 3.24/2.18
API Business up 17.2%
Formulations 15.0%
Alivira award best Company in AH India/ME/Africa



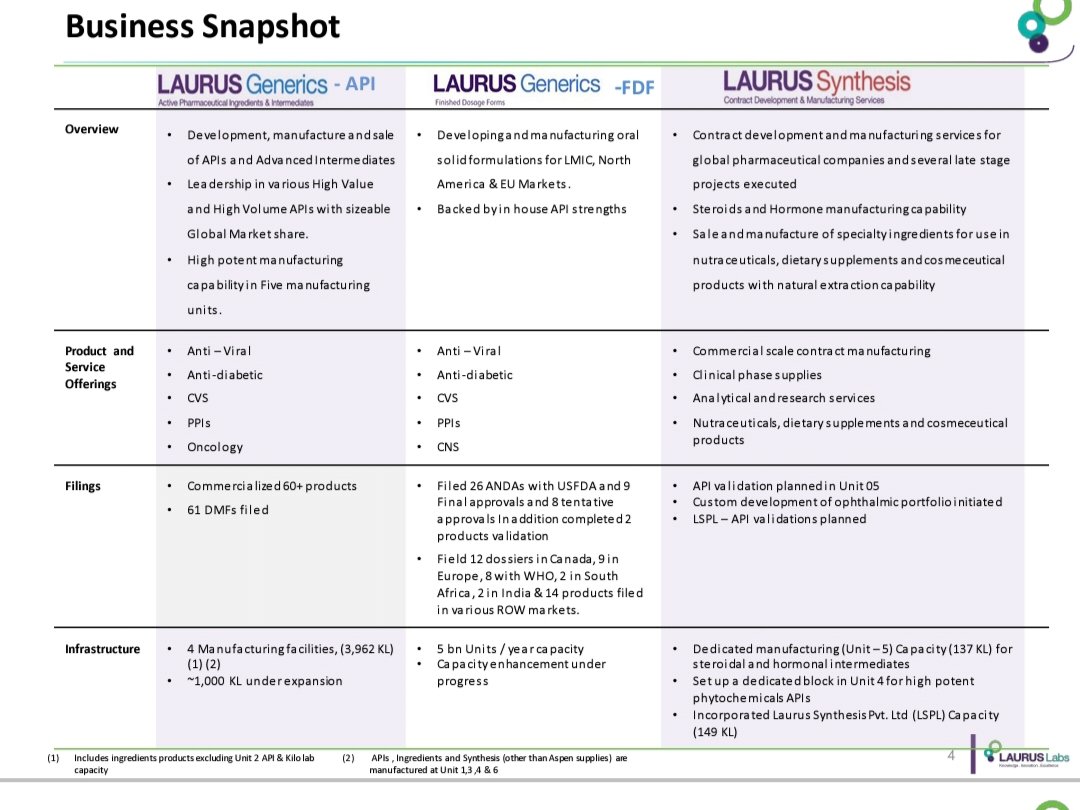
Formulations
EU subdued due operational challenges of Covid
Spain & Germany impacted while Benelux & Sweden reported strong growth
Growth to accelerate with recent launches of CitramoxLA & Halofusol. Tulathromycin launch to reflect from the current quarter
EU subdued due operational challenges of Covid
Spain & Germany impacted while Benelux & Sweden reported strong growth
Growth to accelerate with recent launches of CitramoxLA & Halofusol. Tulathromycin launch to reflect from the current quarter
Brazil & Turkey grow strongly driven by mkt share gain existing portfolio & new launches
India business doubled last 9 months
Integration Zoetis portfolio completed
API Business
Highest quarterly sales 1,297Mn, growth 20%
1/3rd sales from global Top-10 AH players in 9M
India business doubled last 9 months
Integration Zoetis portfolio completed
API Business
Highest quarterly sales 1,297Mn, growth 20%
1/3rd sales from global Top-10 AH players in 9M

11 CEP & 21 US filings/approvals in total (1 CEP approval and 1 USVMF filing in Q3)
Enhanced capacities at Mahad (complete Q3) , Vizag (to be completed Q4) to drive growth in FY22
Formulations
FDFs 1000+
Mftng facilities 5
Sales to regulated mkt 65%
Mktng presence countries 80+
Enhanced capacities at Mahad (complete Q3) , Vizag (to be completed Q4) to drive growth in FY22
Formulations
FDFs 1000+
Mftng facilities 5
Sales to regulated mkt 65%
Mktng presence countries 80+
APIs
Commercial APIs 27
Mftg facilities 3
Sales to regulated mkts 75%
Asset turnover ratio 2.5x
Formulations
Products under development 35+
R&D centers 4
Injectables 38%
Filings in US next 3 yrs 10
APIs
Mols in pipeline 14+
R&D center 1
US filings 21
CEP approvals 11
Commercial APIs 27
Mftg facilities 3
Sales to regulated mkts 75%
Asset turnover ratio 2.5x
Formulations
Products under development 35+
R&D centers 4
Injectables 38%
Filings in US next 3 yrs 10
APIs
Mols in pipeline 14+
R&D center 1
US filings 21
CEP approvals 11
Overall Business Grew 15.1% CC during the quarter; strong growth across both APIs and Formulations
API Business grew 17.2%
Formulations 15.0%
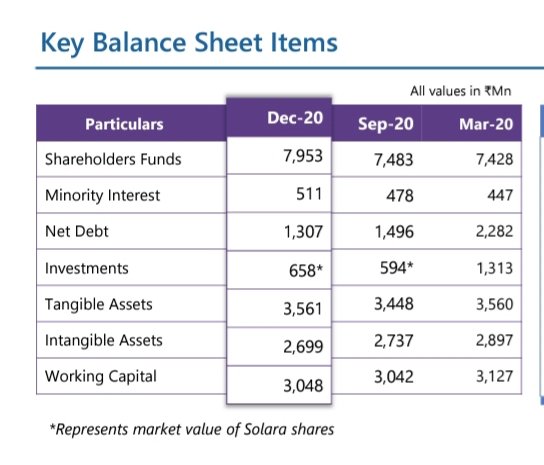
Balance sheet highlights
Cash flow generation 1.5Bn+
Net debt reduction 975Mn in 9M
API Business grew 17.2%
Formulations 15.0%
Balance sheet highlights
Cash flow generation 1.5Bn+
Net debt reduction 975Mn in 9M
Prepayment of all INR denominated term loans of ₹1,250Mn will lead to substantial interest savings going forward
Consolidation of minority interest in Turkey and Netherlands completed
Driving growth in consonance with continued focus on Working capital
Consolidation of minority interest in Turkey and Netherlands completed
Driving growth in consonance with continued focus on Working capital
Unroll
@threadreaderapp
Compile
@threader_app
#sequentScientific #pharma #cdmo #API #Nifty #animalpharma
@threadreaderapp
Compile
@threader_app
#sequentScientific #pharma #cdmo #API #Nifty #animalpharma

• • •
Missing some Tweet in this thread? You can try to
force a refresh