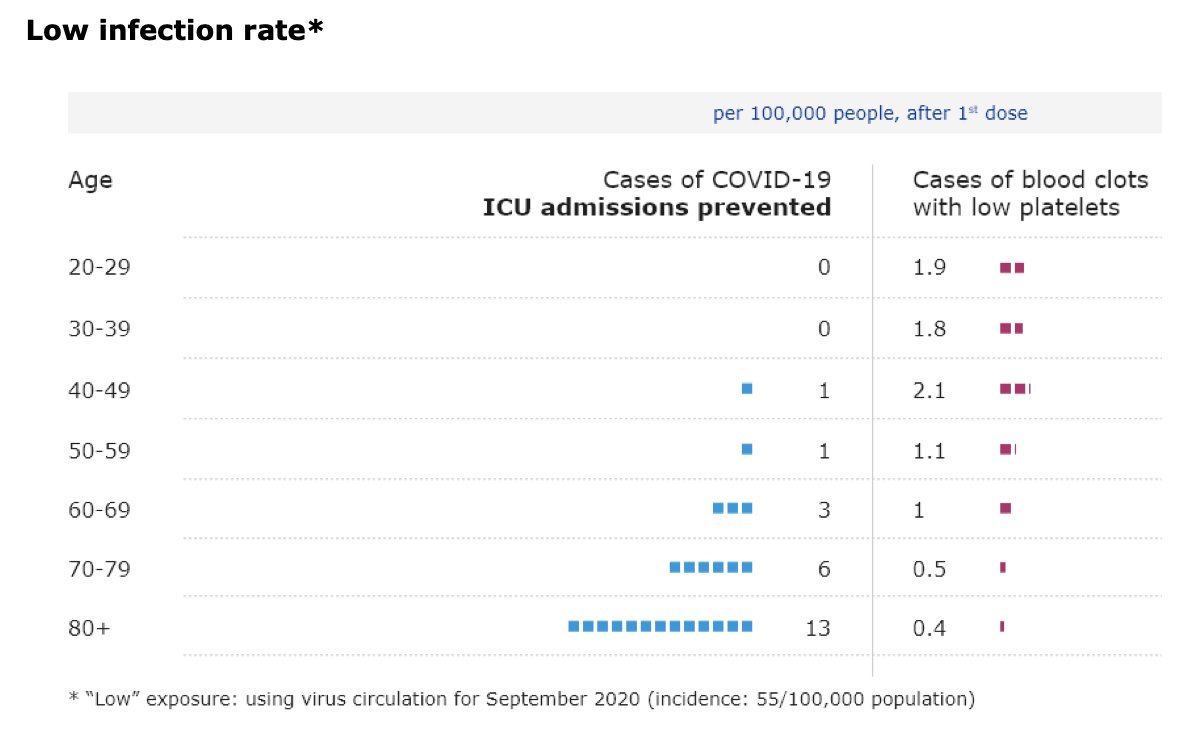
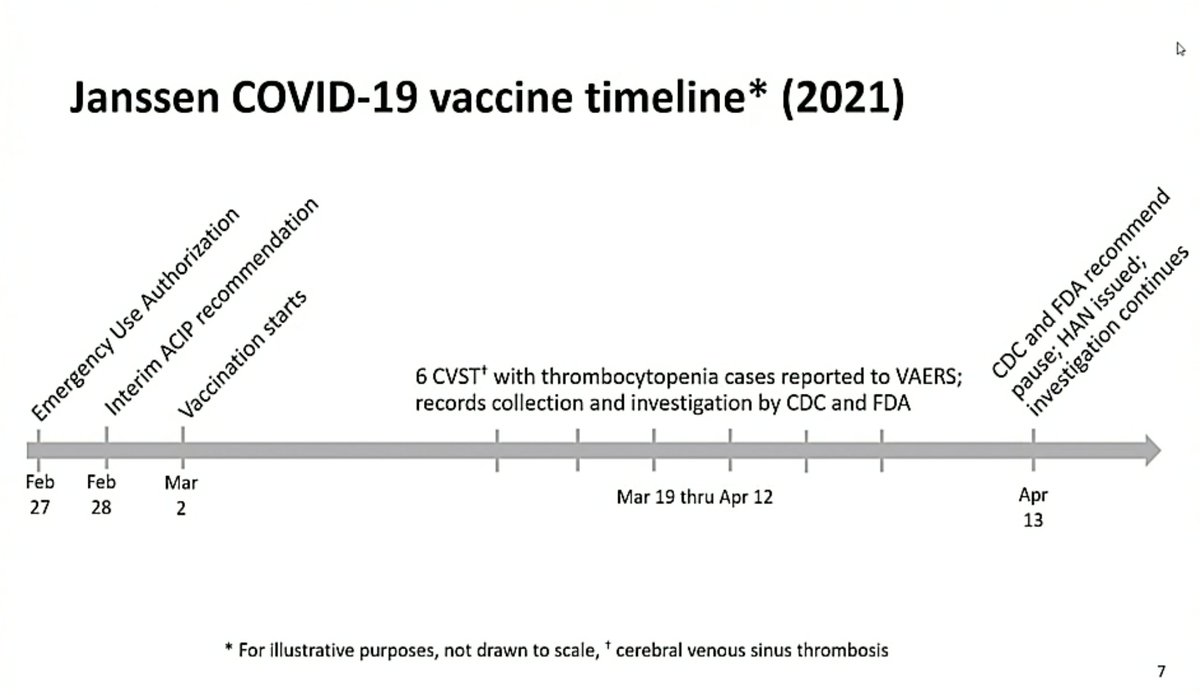
In the US the Advisory Committee on Immunization Practices (ACIP) is meeting at the moment to discuss data on the rare clotting disorders seen after immunization with J&J’s #covid19 vaccine and to make recommendations on future use of the vaccine.
I’ll tweet along a bit.
I’ll tweet along a bit.
Outcomes from the rare clotting disorders are likely to improve from "recognition among physicians also recognition in the public that if you develop a severe headache, severe abdominal pain that you really need to see your doctor", says @mstreif1.
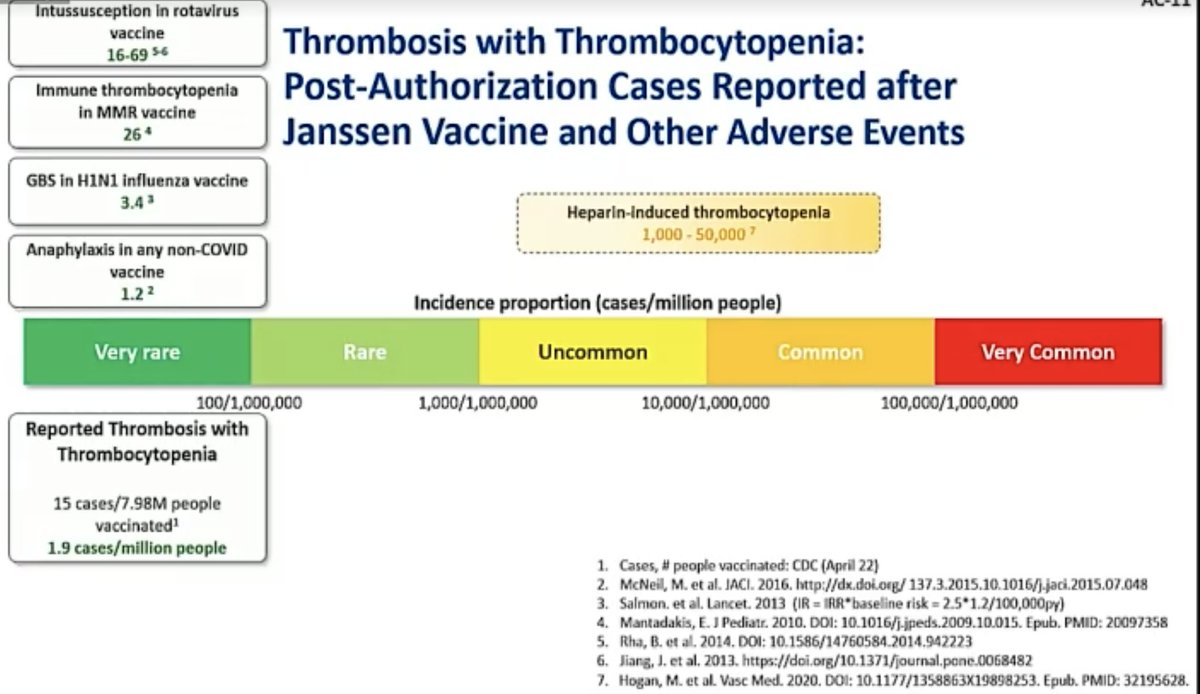
@mstreif1 As of 21 April, 15 confirmed cases of the rare clotting disorder (here called TTS, Thrombosis with Thrombocytopenia Syndrome) after about 8 million vaccinations with J&J’s #covid19 vaccine, says @CDCgov's Tom Shimabukuro 

@mstreif1 @CDCgov Not a single male case even now, though Shimabukuro gives caveats: "there could be cases that simply weren't reported, and there could be cases that become apparent later on, that just had not appeared, but as of now we had zero cases of males."
@mstreif1 @CDCgov Here is the age break-down for the patients and you can see the highest reporting rate is in 30-39 year old women: more than 1 in 100,000. 

@mstreif1 @CDCgov Of the 15 patients, 3 died, 5 have been discharged, 7 remain hospitalised.
(And he adds that none of the patients that died were treated with heparin.)
(And he adds that none of the patients that died were treated with heparin.)

@mstreif1 @CDCgov Shimabakuro summarising now:
“TTS is rare but clinically serious and potentially life threatening and a potentially life threatening adverse event that has been observed in association with the Janssen #COVID19 vaccine"
“TTS is rare but clinically serious and potentially life threatening and a potentially life threatening adverse event that has been observed in association with the Janssen #COVID19 vaccine"

@mstreif1 @CDCgov Discussion now of one report of TTS that was excluded. Showing this just to give an idea of how complex something simple like counting cases can become.
(Patient was PCR-positive for #sarscov2 and at first hospitalisation did not have thrombocytopenia)
(Patient was PCR-positive for #sarscov2 and at first hospitalisation did not have thrombocytopenia)

@mstreif1 @CDCgov If you’re wondering what's happening at ACIP:
It’s time for public comments at the moment and that means there is a lot of anti-vaxxers commenting but also people arguing the pause was wrong.
Everyone gets 3 minutes.
It’s time for public comments at the moment and that means there is a lot of anti-vaxxers commenting but also people arguing the pause was wrong.
Everyone gets 3 minutes.
@mstreif1 @CDCgov Small break now.
Meeting continues in 15 minutes with presentation from J&J representative.
Meeting continues in 15 minutes with presentation from J&J representative.
@mstreif1 @CDCgov “We believe that the excellent efficacy we nevertheless maintain against the variants is in part due to the T cell immunity that recognizes so many epitopes of the spike protein.”, says @mmammen, global head of R&D at Janssen, making the case for the shot. 

@mstreif1 @CDCgov @mmammen "Our vaccine is the only authorized vaccine that uses a single dose regimen. The benefits of this dosing cannot be overstated “, says @mmammen. “These features of the vaccine allow vaccination of people that might otherwise not be able to, or choose not to get a shot." 
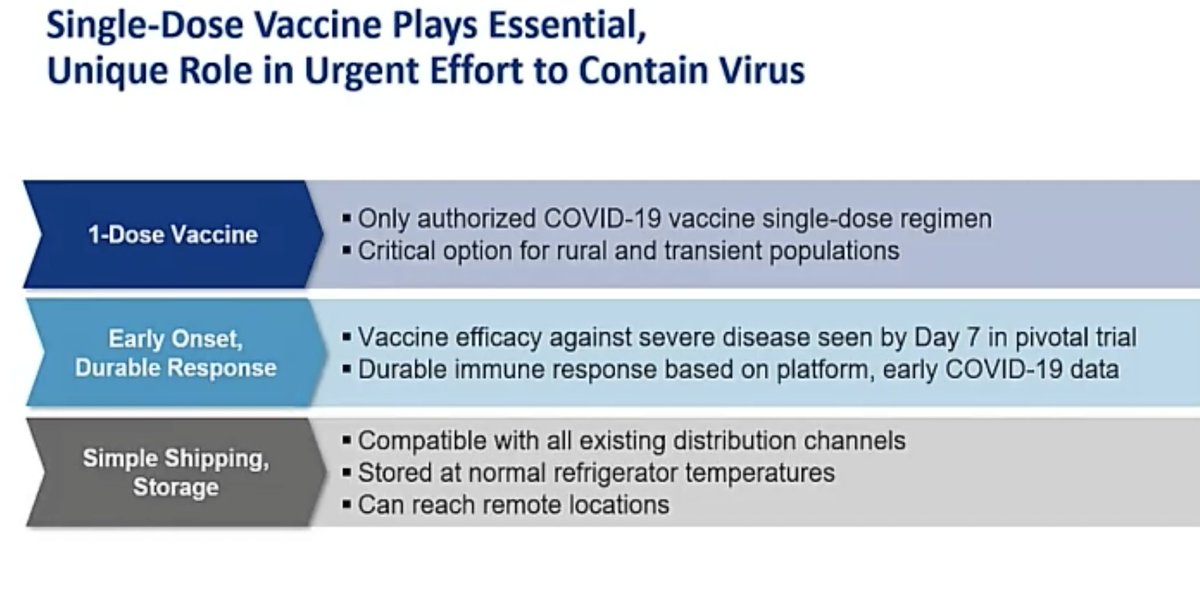
@mstreif1 @CDCgov @mmammen “Our analysis of five large observational US healthcare databases that included over 16 million people estimated the background rate of thrombosis CVST with thrombocytopenia to be approximately 0.1 cases per million.”, says Joanne Waldstreicher, chief medical officer at J&J. 
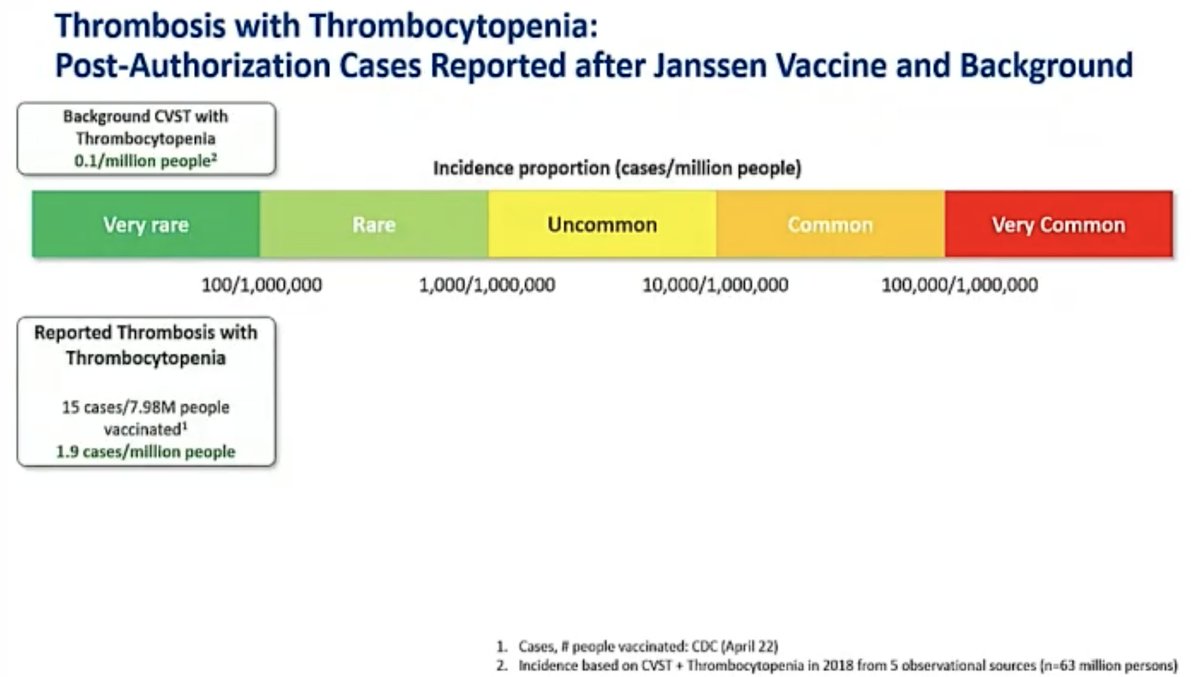
@mstreif1 @CDCgov @mmammen Here is her analysis of risks and benefits of this vaccine in general population and in women younger than 50: 


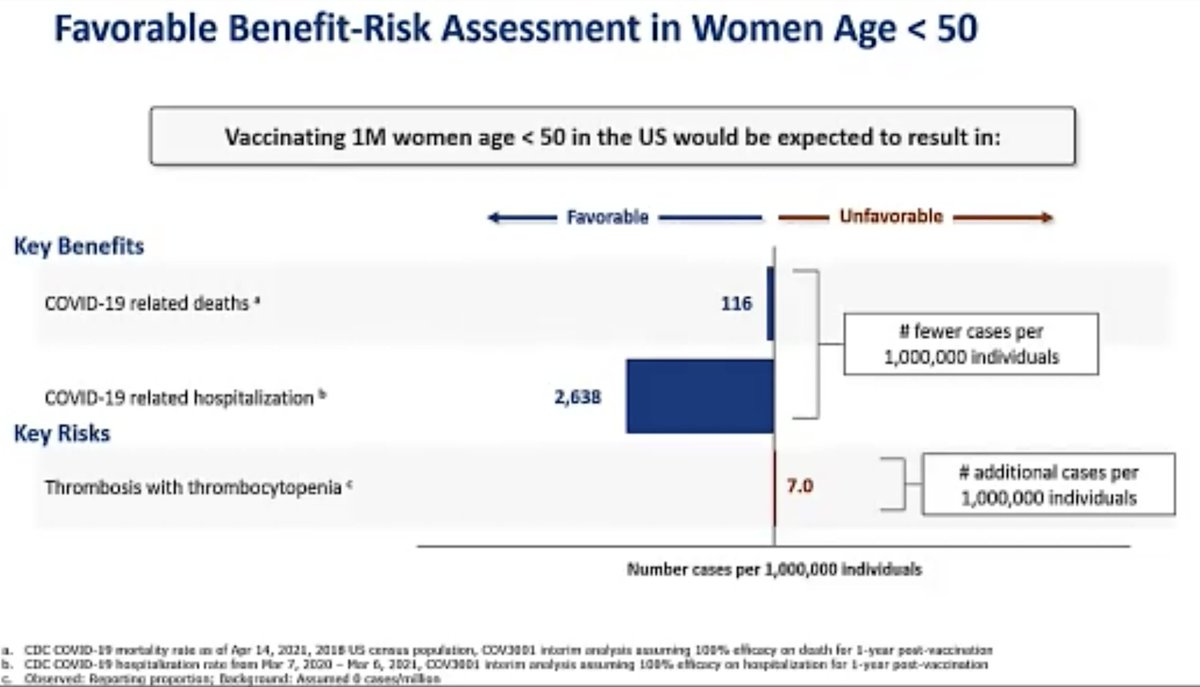
@mstreif1 @CDCgov @mmammen “We absolutely agree with the FDA on the implementation of a warning within our label and patient and physician factsheets describing this very rare event, including how it can be identified early and diagnosed and treated”.
Here is the agreed on warning:
Here is the agreed on warning:

@mstreif1 @CDCgov @mmammen “A restriction in use here could have a negative impact on the success of achieving global herd immunity, both here in the US and globally,” says Waldstreicher.
“The pandemic will not be over until a large segment of the global population is vaccinated."
“The pandemic will not be over until a large segment of the global population is vaccinated."
@mstreif1 @CDCgov @mmammen Q whether other adenovirus vector vaccines (like the Ebola one) have shown similar side effects
“We today have more than 200,000 people vaccinated with Ebola vaccine, and we have not observed any of this phenomenon in that particular clinical trial setting”, says someone from J&J
“We today have more than 200,000 people vaccinated with Ebola vaccine, and we have not observed any of this phenomenon in that particular clinical trial setting”, says someone from J&J
@mstreif1 @CDCgov @mmammen “The risk of TTS appears highest in females less than 50 years of age, and other risk factors for TTS are not yet well established”, says Grace Lee of Vaccine Safety technical work group and runs through risk mitigation strategies: 



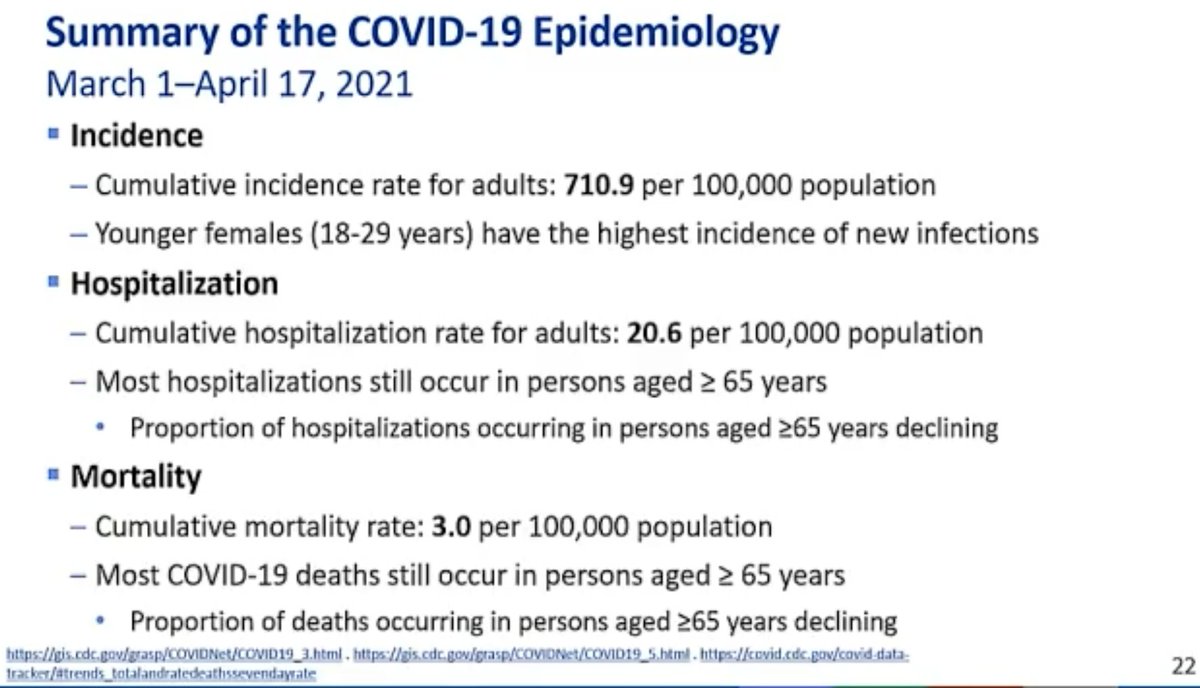
@mstreif1 @CDCgov @mmammen Interesting data as CDC’s Sara Oliver runs through the public health aspects of the risk/benefit analysis:
Half of cases are #b117.
Highest rate of infections in US currently in women 18-29 years old.

Half of cases are #b117.
Highest rate of infections in US currently in women 18-29 years old.
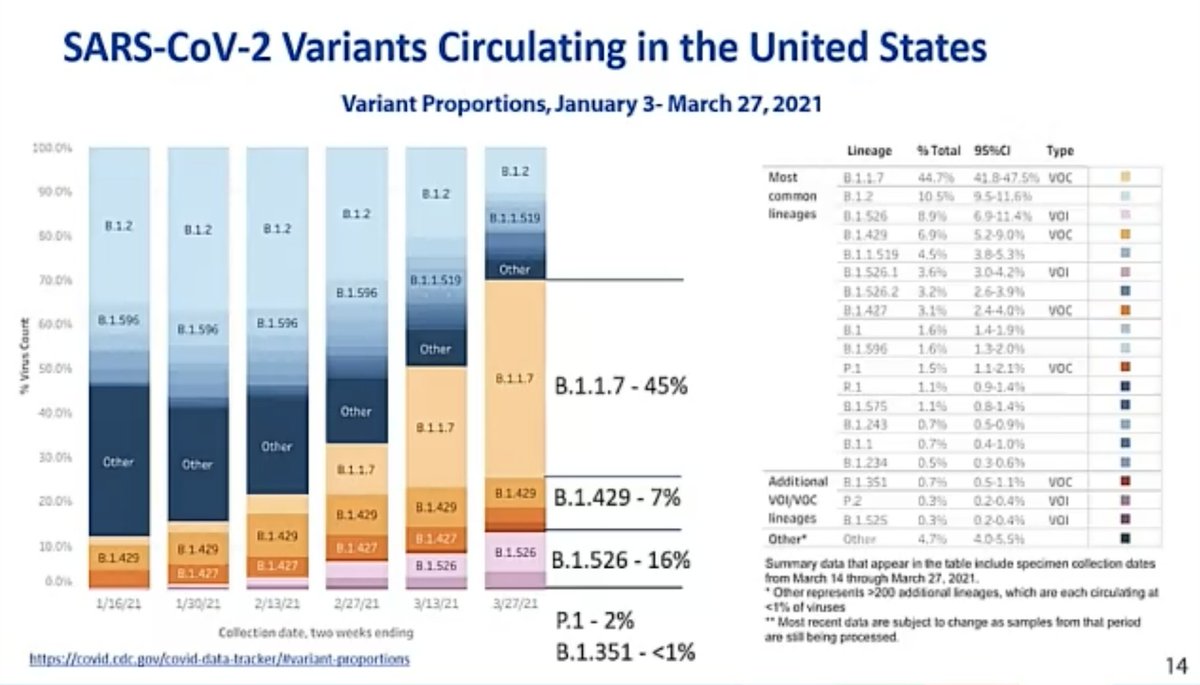
@mstreif1 @CDCgov @mmammen “CVST can occur after #COVID19, although it is very rare, in less than 0.1% of hospitalized COVID patients. Translating this to overall numbers, it could be estimated to be around five to six cases of CVST per million #sarscov2 infections”, says Oliver. 
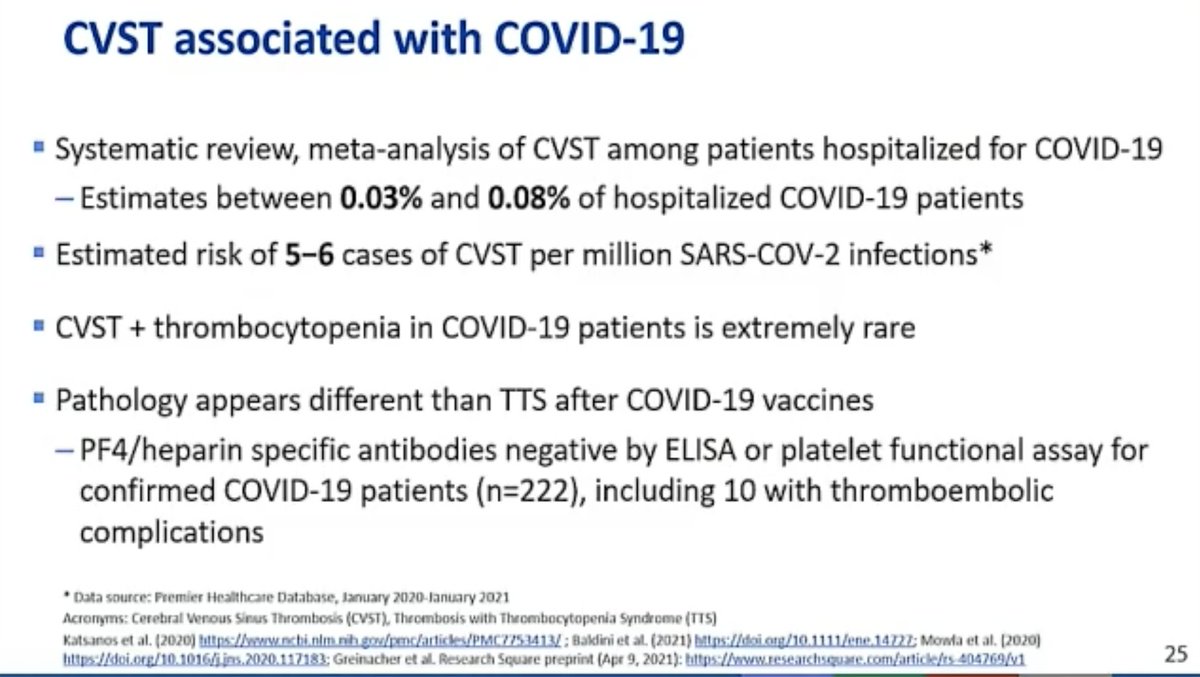
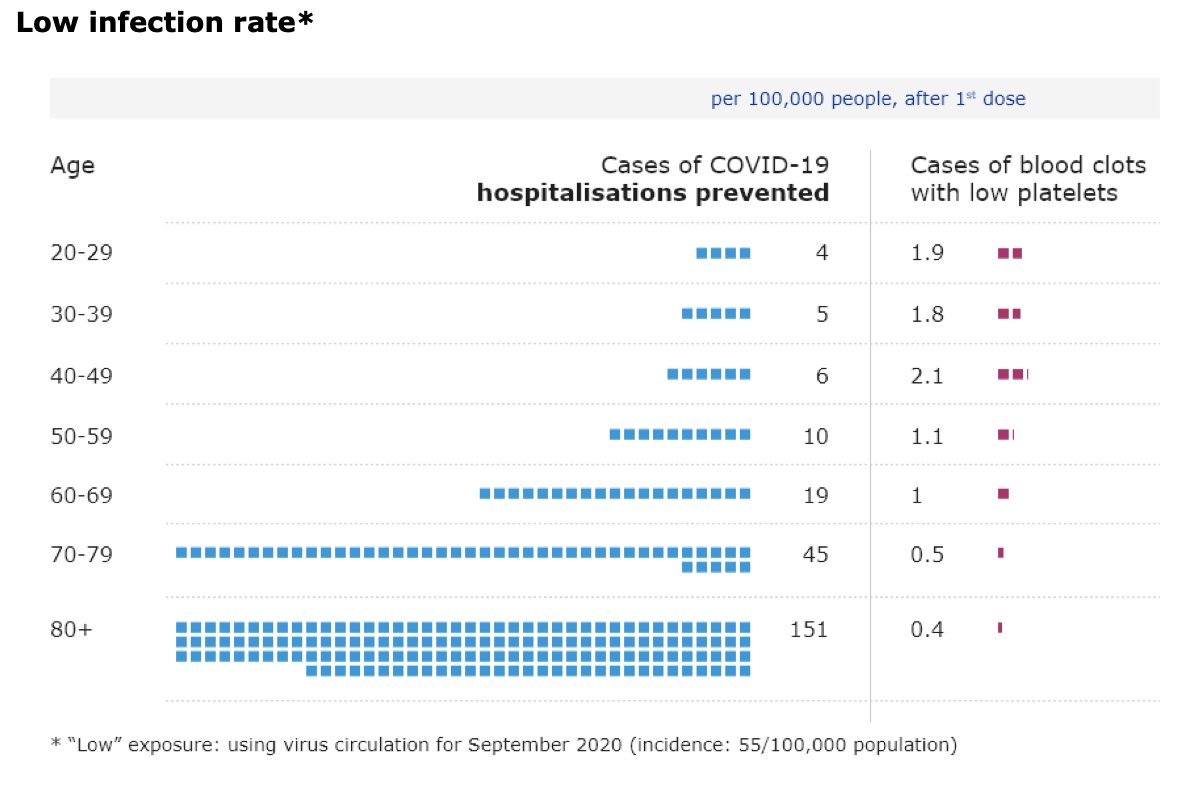
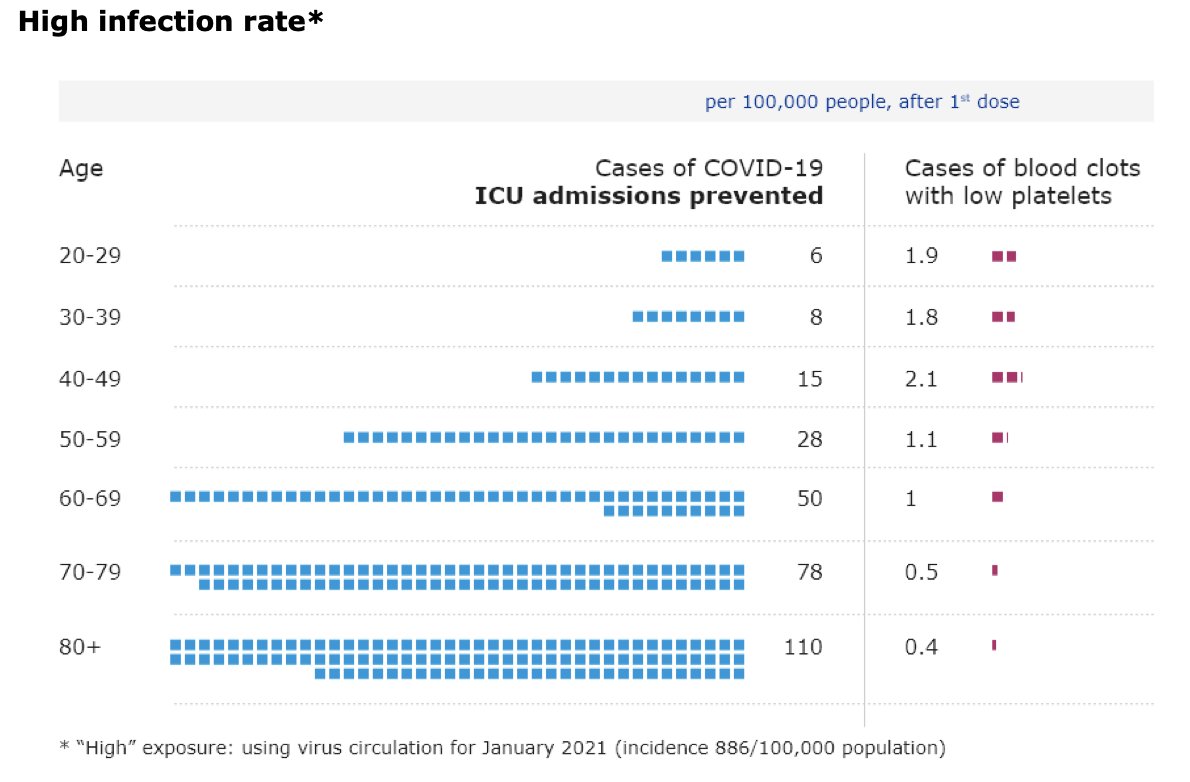
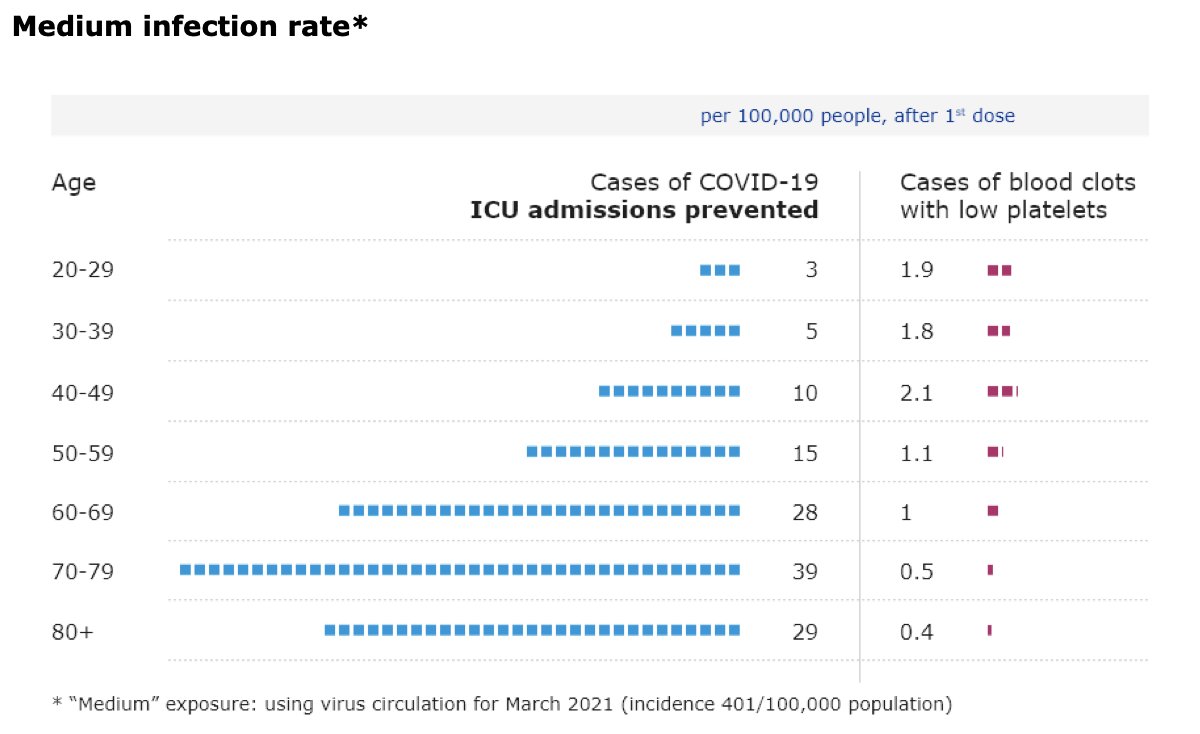
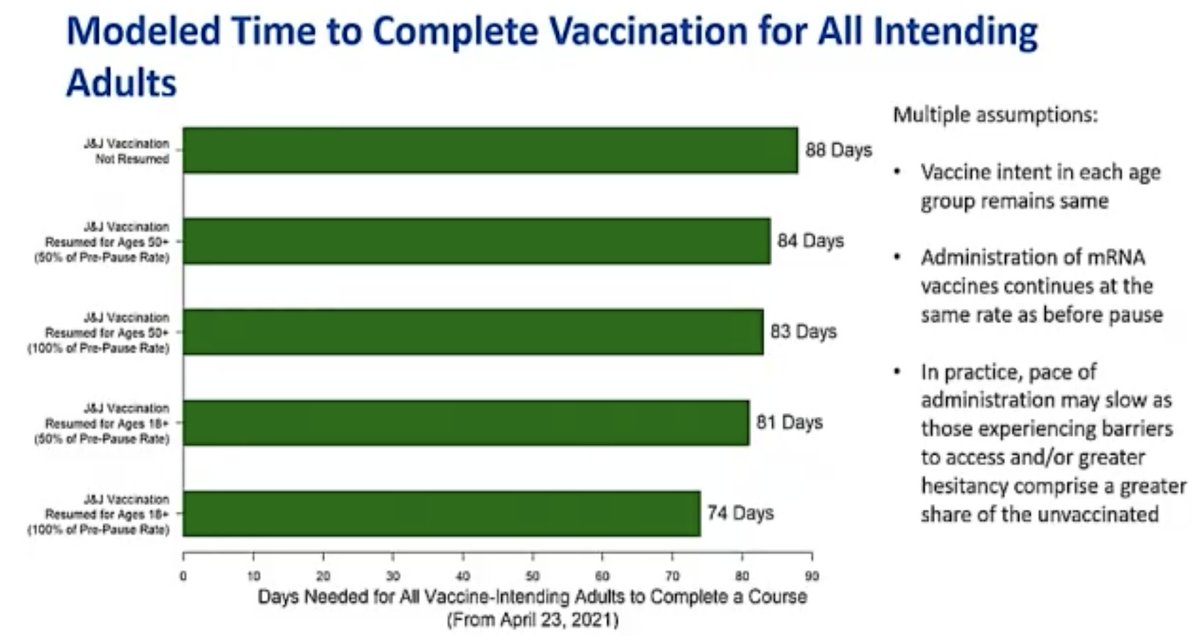
@mstreif1 @CDCgov @mmammen Oliver is presenting outcomes of modeling risk-benefit at the population level (over 6 months) using 5 different scenarios.
Fascinating that not using J&J vaccine only adds 2 weeks to time until everyone who wants is vaccinated.
Fascinating that not using J&J vaccine only adds 2 weeks to time until everyone who wants is vaccinated.
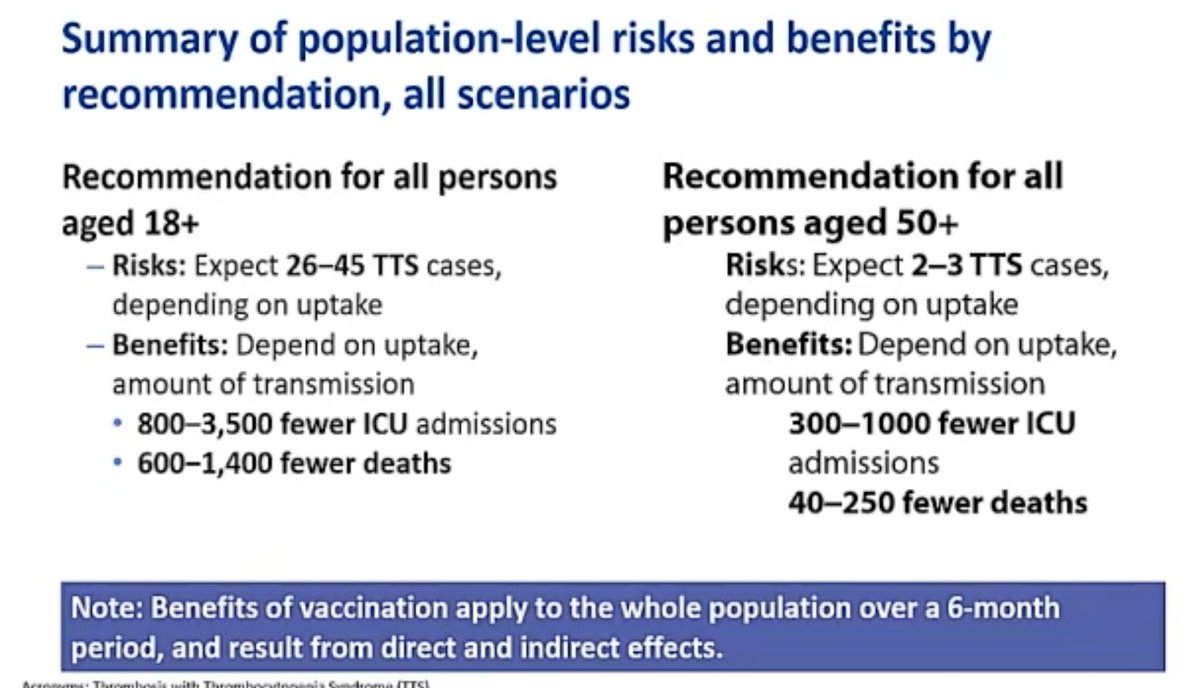
@mstreif1 @CDCgov @mmammen So this is the comparison on a population level of risks and benefits of restarting the J&J vaccine in all adults vs. those over 50 years of age.
Take-home:
Restricting vaccine to 50+ prevents a few dozen TTS cases, but leads to hundreds more deaths from #covid19.

Take-home:
Restricting vaccine to 50+ prevents a few dozen TTS cases, but leads to hundreds more deaths from #covid19.

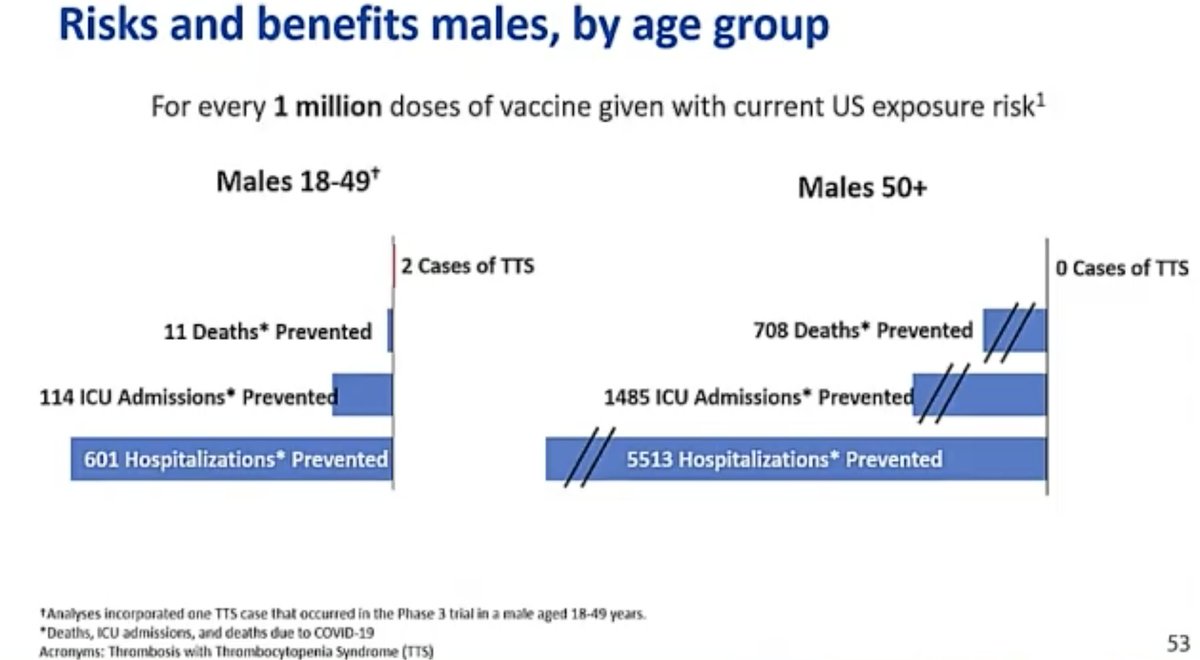
@mstreif1 @CDCgov @mmammen And this is risk-benefit analysis on an individual level broken down for men and women by age: 


@mstreif1 @CDCgov @mmammen Now talking about acceptance of the vaccine and feasibility of using it only in specific populations.
(Pretty amazing level of detail in this discussion of all the repercussions, risk benefit calculations.)
(Pretty amazing level of detail in this discussion of all the repercussions, risk benefit calculations.)

@mstreif1 @CDCgov @mmammen “Jurisdictions continue to mention four populations at risk of disproportionate impact: persons experiencing homelessness,
homebound populations,
incarcerated individuals and migrant or seasonal populations”, says Oliver.
homebound populations,
incarcerated individuals and migrant or seasonal populations”, says Oliver.

@mstreif1 @CDCgov @mmammen “It was felt that if the Janssen vaccine were no longer available these populations may be disproportionately impacted.”
(This is a really important point for the discussion!)
(This is a really important point for the discussion!)
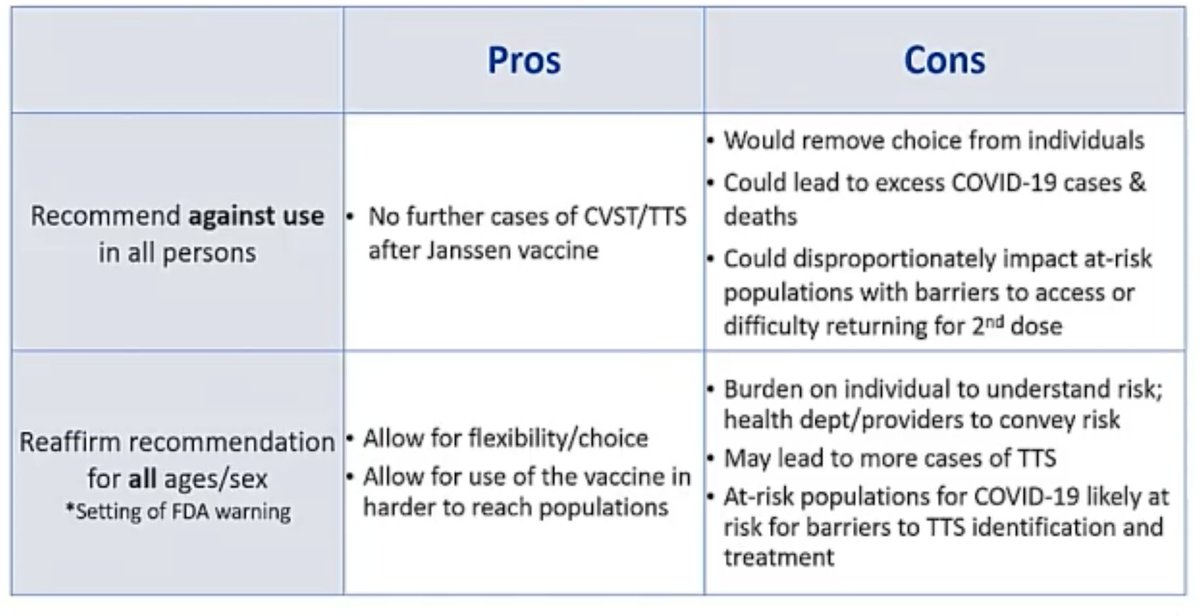
@mstreif1 @CDCgov @mmammen So, the policy options:
- recommend against use
- reaffirm use for everyone
- recommend for people older than 50
- recommend for everyone, but allow women to choose a different vaccine
- recommend against use
- reaffirm use for everyone
- recommend for people older than 50
- recommend for everyone, but allow women to choose a different vaccine

@mstreif1 @CDCgov @mmammen Some of the pros and cons of these four options here:
Seems clear that the last option has a lot going for it.

Seems clear that the last option has a lot going for it.

@mstreif1 @CDCgov @mmammen So:
Given the review of the benefits and risks, what recommendation does ACIP feel is appropriate for use of the Janssen Covid-19 vaccine?
Discuss.
Given the review of the benefits and risks, what recommendation does ACIP feel is appropriate for use of the Janssen Covid-19 vaccine?
Discuss.

@mstreif1 @CDCgov @mmammen Q how feasible option 4 is, ie can different vaccines be available?
Yes, says @nirav_mainecdc, but it might slow things down a little bit. And it does introduce a small potential for errors.
Yes, says @nirav_mainecdc, but it might slow things down a little bit. And it does introduce a small potential for errors.
@mstreif1 @CDCgov @mmammen @nirav_mainecdc "We should also consider the impact on immunocompromised patients”, says @KottonNelson. Vaccinating the population as fast as possible would more rapidly free up additional doses to try and give boosters to these patients who are not responding to the vaccine, she says.
@mstreif1 @CDCgov @mmammen @nirav_mainecdc @KottonNelson “I think it's really important that when we talk about this, that we do include gender”, says Helen Keipp Talbot.
"I don't think that we can ignore the fact that the majority of the cases have happened in women"
"I don't think that we can ignore the fact that the majority of the cases have happened in women"
@mstreif1 @CDCgov @mmammen @nirav_mainecdc @KottonNelson Good point from @DocJeffD, that as the risk of #covid19 recedes the risk-benefit-equation will change and that the recommendations may need to be updated then.
@mstreif1 @CDCgov @mmammen @nirav_mainecdc @KottonNelson @DocJeffD "I'm concerned that without a strong statement from ACIP, that that decision could contribute to vaccine hesitancy, and that any contribution to vaccine hesitancy is far more likely to cause real harm to people”, says Robert Gluckman arguing for a strong statement about benefits
@mstreif1 @CDCgov @mmammen @nirav_mainecdc @KottonNelson @DocJeffD Main discussion point seems to be how feasible option 4 is and whether it might increase vaccine hesitancy.
Still assume that is where we will end up.
Still assume that is where we will end up.
@mstreif1 @CDCgov @mmammen @nirav_mainecdc @KottonNelson @DocJeffD “The last 11 days to me have been reassuring in that we haven't identified hundreds more cases despite enhanced awareness and case finding across the US, which was actually my worry last week”, says Grace Lee. (Important point!)
Says she would have decided differently last week.
Says she would have decided differently last week.
@mstreif1 @CDCgov @mmammen @nirav_mainecdc @KottonNelson @DocJeffD "I do not think that that fourth recommendation falls within what we would normally consider as a recommendation per our usual framework”, says Lee. Says that option could be confusing and advocates for option 2.
The discussion was a fascinating mix ranging from the potential implications of restricting the vaccine recommendation on vaccine equity to the order in which the symptoms should be listed in the fact sheet...
Now moving to vote on option 2.
The motion:
The Janssen Covid-19 vaccine is recommended for persons 18 years of age and older in the US population under the FDA’S Emergency Use Authorization.
The Janssen Covid-19 vaccine is recommended for persons 18 years of age and older in the US population under the FDA’S Emergency Use Authorization.

The vote is:
10 in favor
4 opposed
1 abstention
10 in favor
4 opposed
1 abstention
So that's that.
The recommendation is to unpause immunizations with the J&J vaccine for everyone.
(Not what I would have guessed a few hours ago)
The recommendation is to unpause immunizations with the J&J vaccine for everyone.
(Not what I would have guessed a few hours ago)
Pablo Sanchez, who voted no, explains that he has no problem with continued availability of the vaccine but feels that stronger language is needed in order to "make sure that people are informed appropriately”.
“I am very sorry that we haven't chosen to put up front the knowledge that we have, that this is unique, it's clustered, it's almost certainly related to the vaccine, and there are options", says Sarah Long.
“I just want to emphasize that I actually agree with all of my colleagues who voted no.
I absolutely think this is a serious adverse event, we need to continue to ensure that awareness is raised,”, says Grace Lee. Option 4 would have been confusing she says.
I absolutely think this is a serious adverse event, we need to continue to ensure that awareness is raised,”, says Grace Lee. Option 4 would have been confusing she says.
And the meeting’s adjourned. Right on time.
11 pm here in Berlin. So I’m off.
11 pm here in Berlin. So I’m off.
• • •
Missing some Tweet in this thread? You can try to
force a refresh