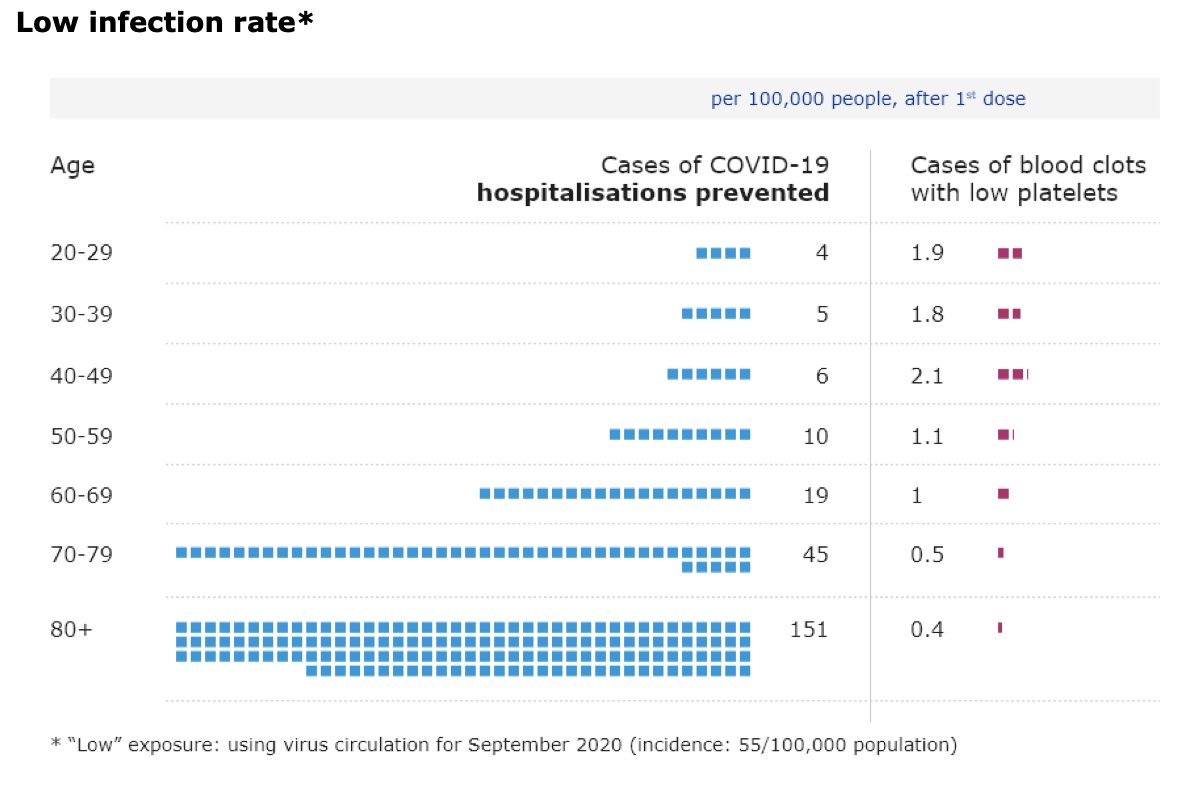
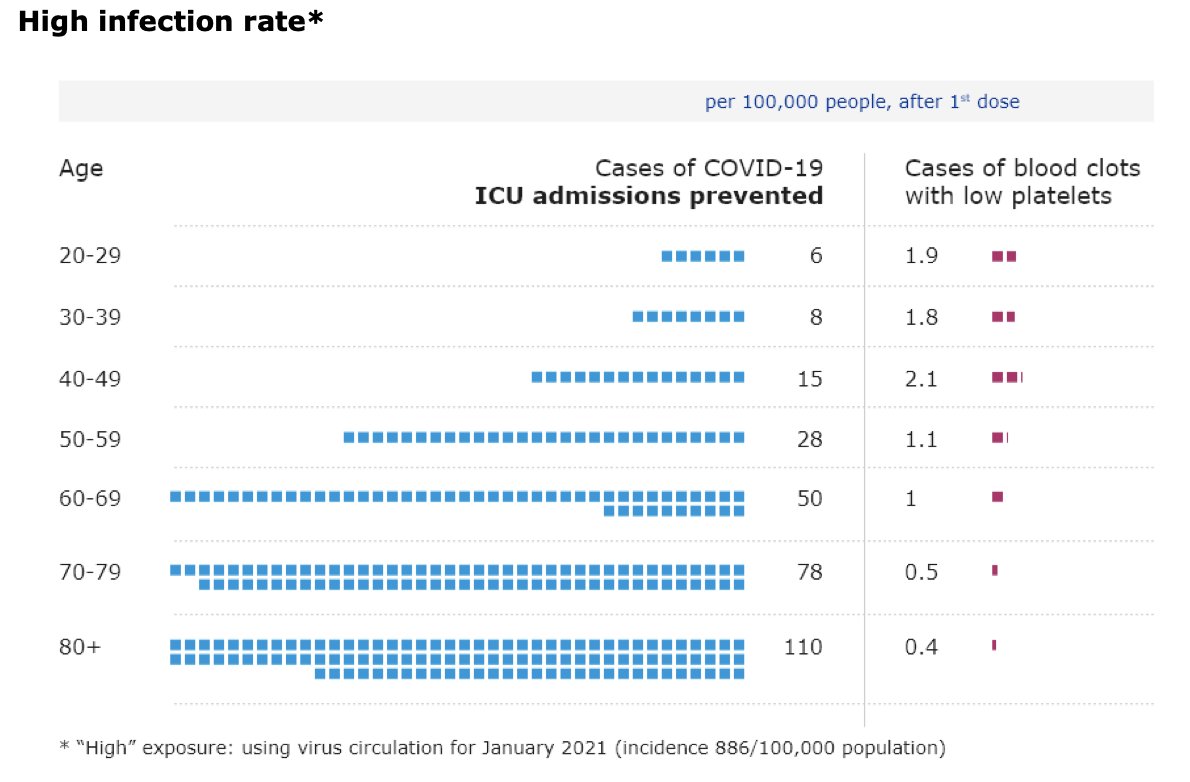
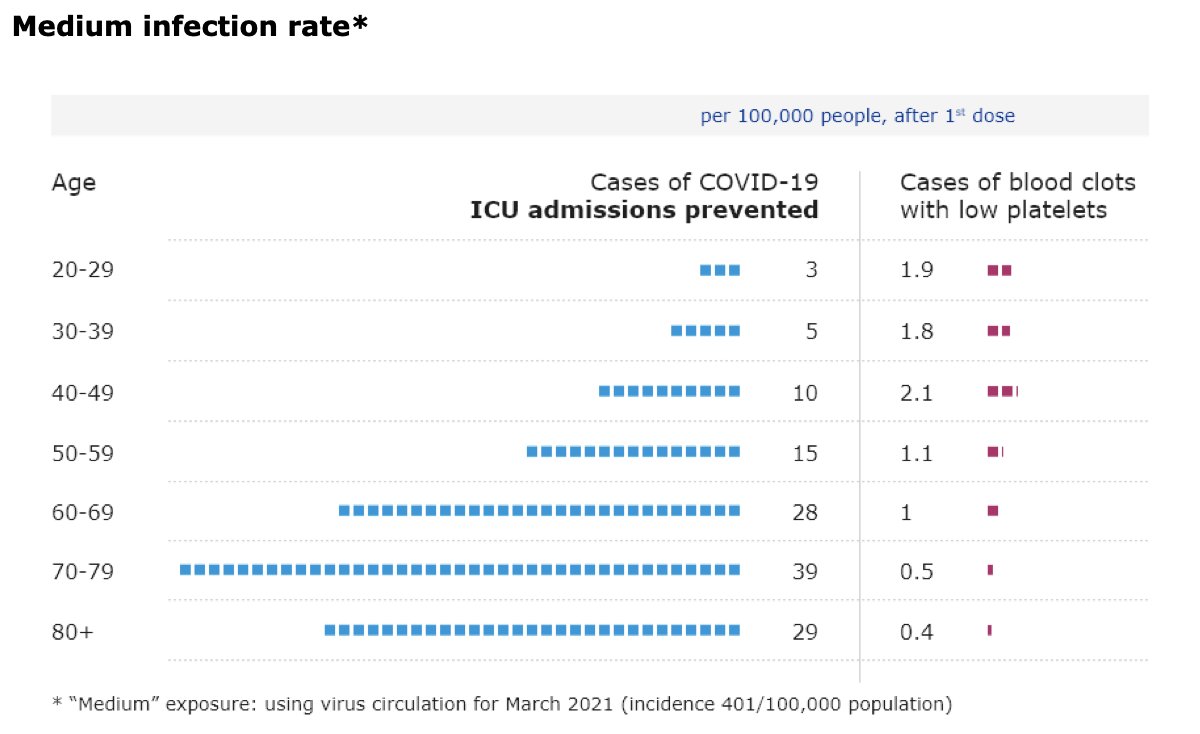
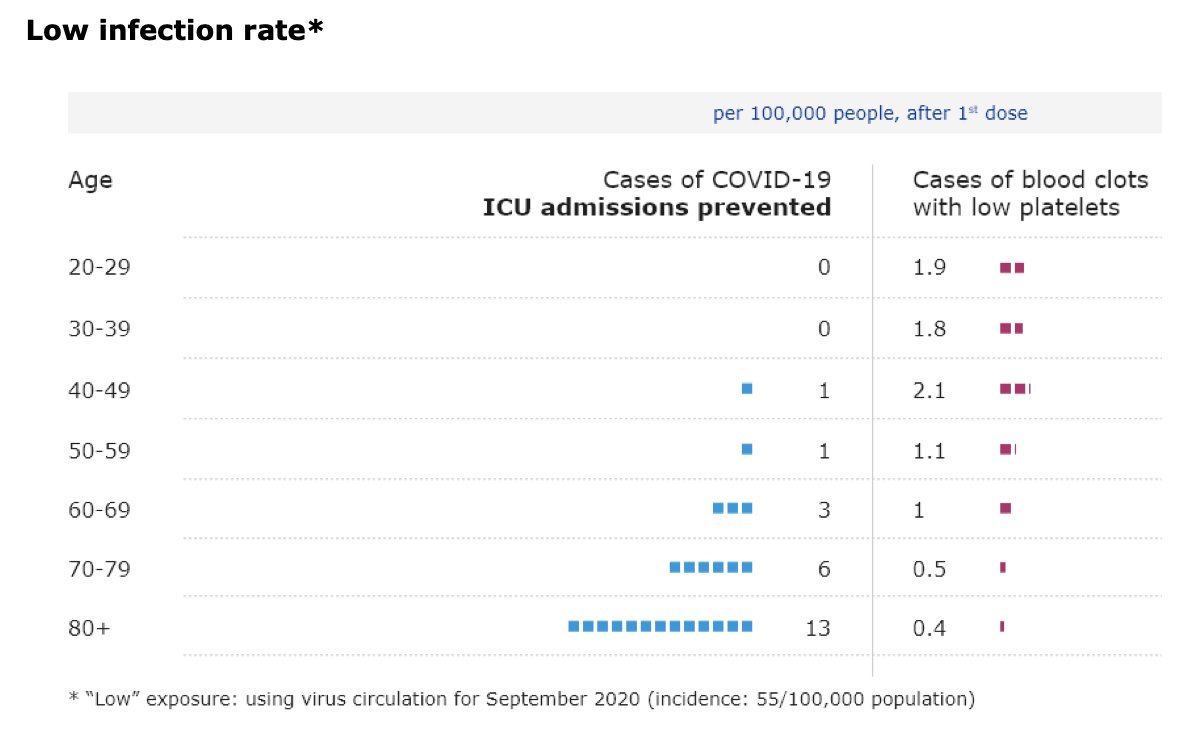
UK’s Joint Committee on Vaccination and Immunisation is now advising healthy adults younger than 40 “to receive an alternative to the Oxford/AstraZeneca vaccine – where available and only if this does not cause substantial delays in being vaccinated”.
gov.uk/government/new…
gov.uk/government/new…
This is based on the fact that “the chances of a younger person becoming seriously ill with #COVID19 get smaller as infection rates increasingly come under control in the UK”.
@GretchenVogel1 and explored these trade-offs in risk and benefit here:
sciencemag.org/news/2021/05/w…
@GretchenVogel1 and explored these trade-offs in risk and benefit here:
sciencemag.org/news/2021/05/w…
@GretchenVogel1 UK's regulator, the MHRA:
"The balance of benefits and risks is very favourable for older people but is more finely balanced for younger people and we advise that this evolving evidence should be taken into account when considering the use of the vaccine”
gov.uk/government/new…
"The balance of benefits and risks is very favourable for older people but is more finely balanced for younger people and we advise that this evolving evidence should be taken into account when considering the use of the vaccine”
gov.uk/government/new…
@GretchenVogel1 Emerging evidence suggests that second dose of AZ is not associated with the same amount of clotting disorders haematologist Beverly Hunt said just now in @SMC_London briefing. Some similar cases, but apparently not a single one with anti-PF4 antibodies so far.
• • •
Missing some Tweet in this thread? You can try to
force a refresh