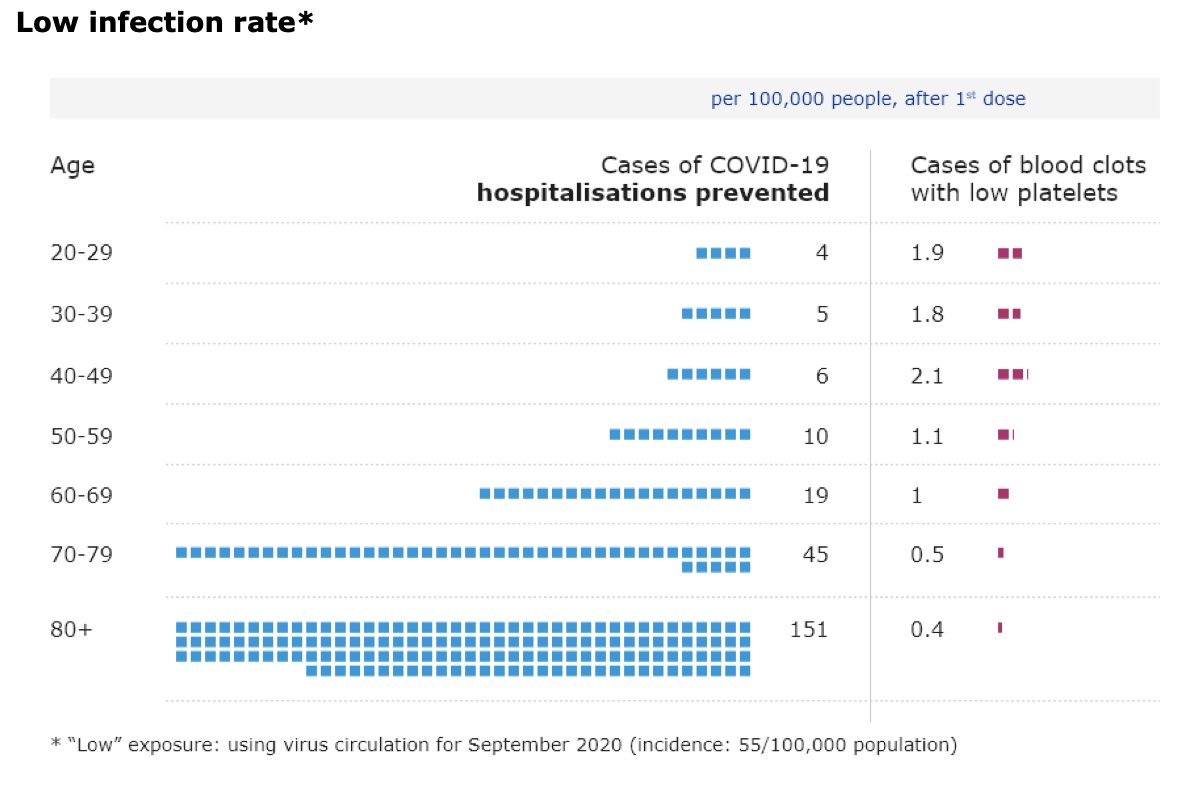
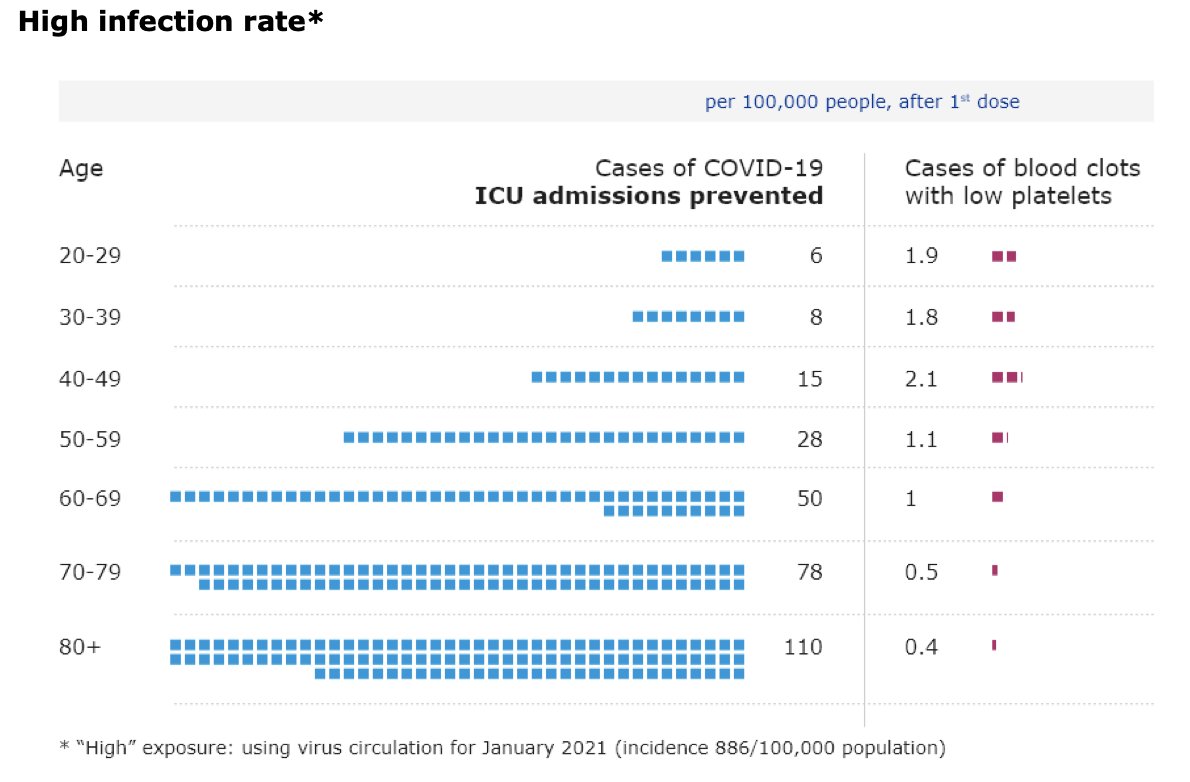
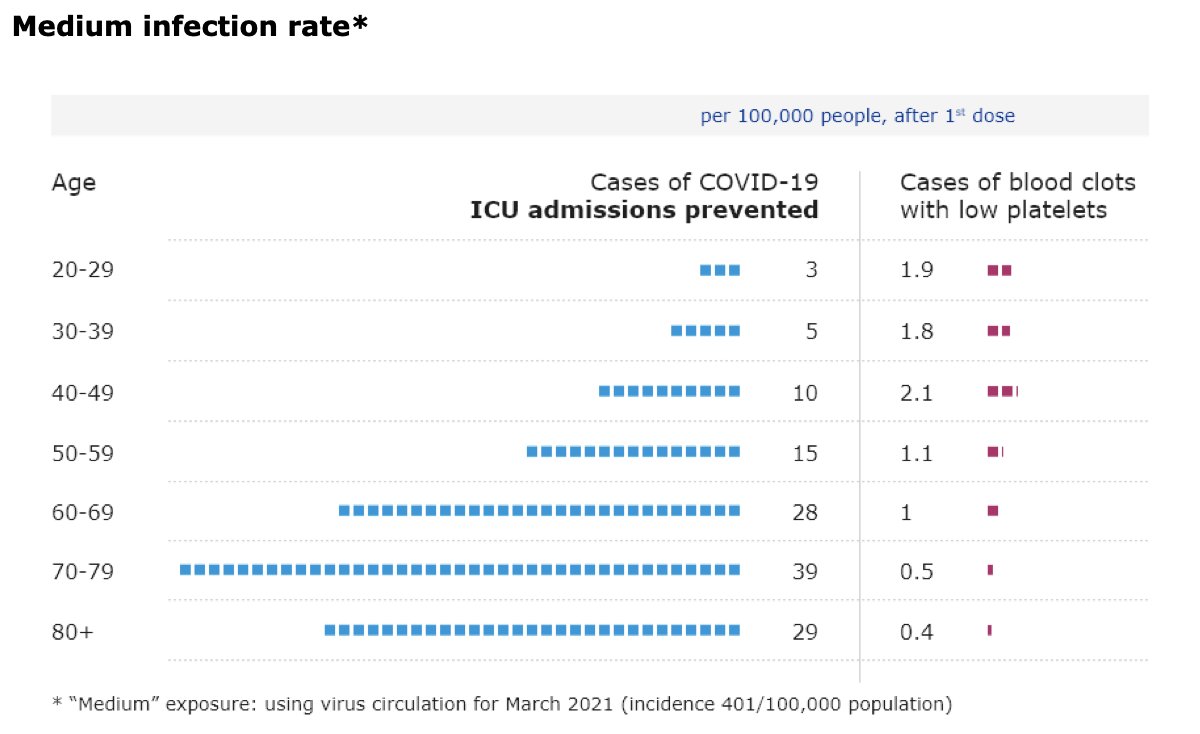
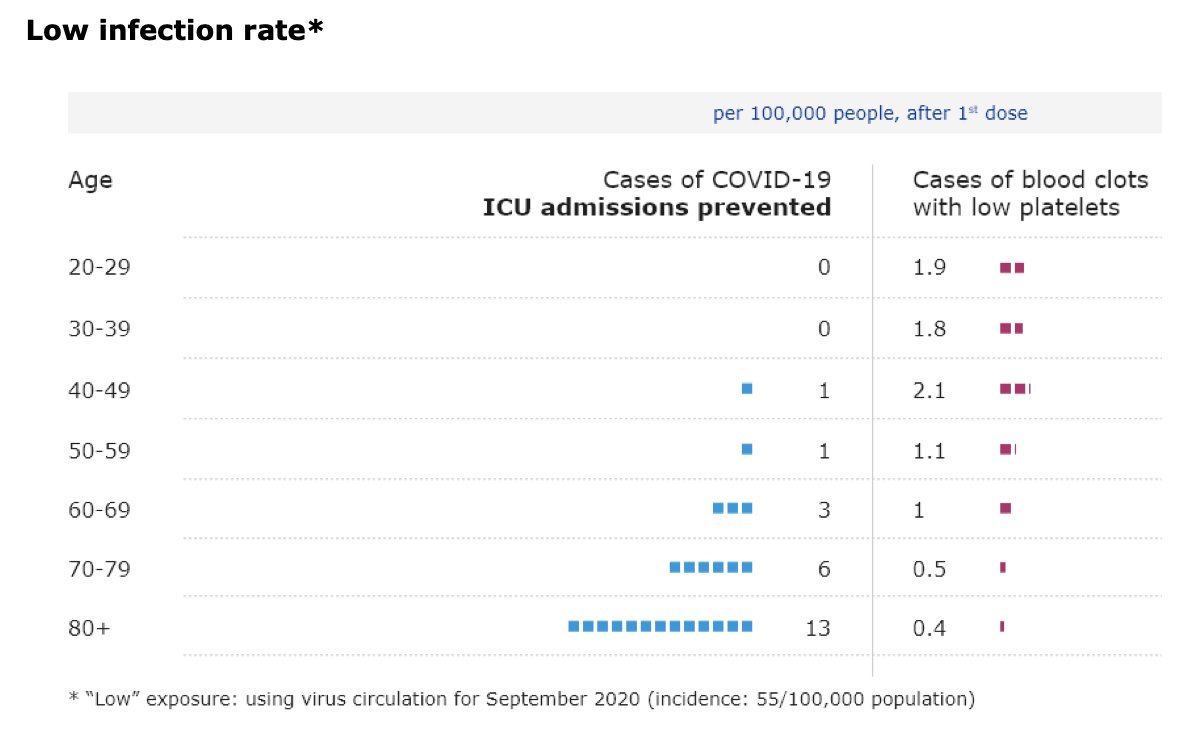
“I know that this is not a politically easy thing to do. So I very much appreciate the leadership of the United States", says @DrTedros at @WHO presser on #covid19 about US support for #TRIPSwaiver “We urge other countries to follow their example.”
@DrTedros @WHO "We are in an unprecedented crisis that requires unprecedented action”, says @drtedros.
“The World Trade Organization provisions for IP waivers were designed precisely for a situation like this.
If we don't use them now, then when?"
“The World Trade Organization provisions for IP waivers were designed precisely for a situation like this.
If we don't use them now, then when?"
@DrTedros @WHO This is important news:
"This afternoon, WHO gave emergency use listing to Sinopharm Beijing's #COVID19 vaccine, making it the sixth vaccine to receive WHO validation for safety, efficacy and quality."
"This afternoon, WHO gave emergency use listing to Sinopharm Beijing's #COVID19 vaccine, making it the sixth vaccine to receive WHO validation for safety, efficacy and quality."
@DrTedros @WHO (Sinopharm’s vaccine is an inactivated virus vaccine. @nytimes has a nice explainer here:
nytimes.com/interactive/20…)
nytimes.com/interactive/20…)
@DrTedros @WHO @nytimes “Fundamentally what we want to do is reverse the usual logic” says @MazzucatoM, chair of @WHO Council on the Economics of Health for All.
Not “invest in health, because it's good for the economy”, but focus on health for all, then “understand how to restructure economy"
Not “invest in health, because it's good for the economy”, but focus on health for all, then “understand how to restructure economy"
@DrTedros @WHO @nytimes @MazzucatoM Q: is there any safe way for Olympics to happen?
"we will leave it to the authorities in Japan who are highly competent to decide what level of attendance could occur in the Olympics”, says @DrMikeRyan. Travel to Olympics from abroad is not on the table, he says.
"we will leave it to the authorities in Japan who are highly competent to decide what level of attendance could occur in the Olympics”, says @DrMikeRyan. Travel to Olympics from abroad is not on the table, he says.
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan “The issue will be the extent to which social mixing and socializing is allowed around the Olympic venues themselves”, says @DrMikeRyan. “Decisions around that level of mixing will be made by the Japanese authorities in due course”, based on epidemiological situation.
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan “The information we have for people over 60 is still very scarce”, says SAGE chair, Alejandro Cravioto on efficacy of Sinopharm vaccine. Still recommended for all people aged 18+. "There is no reason to think that the vaccine would behave differently in this older age group."
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan Q about vaccinating children.
Cravioto: “For the time being, we don't think that there is the vaccine or the actual need to start thinking of this age group as a priority group within the framework that SAGE has designed to see who should be vaccinated and in which sequence."
Cravioto: “For the time being, we don't think that there is the vaccine or the actual need to start thinking of this age group as a priority group within the framework that SAGE has designed to see who should be vaccinated and in which sequence."
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan “We are aware that Canada did authorize the use of the Pfizer vaccine for 12-15 year olds on Wednesday, the first country to provide such an authorization, and we do expect additional authorizations to come through for younger age groups, below the age of 16”, says @Kate_L_OBrien
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan @Kate_L_OBrien “We would really like to re-emphasize that the priority really needs to be getting vaccine to all countries in the world for the highest priority groups before we start advancing to groups that have much lower risk of disease”, says @Kate_L_OBrien
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan @Kate_L_OBrien Q about B.1.617 (variant identified in India)
This variant "is one that we are looking at very, very carefully looking at data that is coming directly from our colleagues in India who are studying this virus variant, as well as studies" in other countries”, says @mvankerkhove
This variant "is one that we are looking at very, very carefully looking at data that is coming directly from our colleagues in India who are studying this virus variant, as well as studies" in other countries”, says @mvankerkhove
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan @Kate_L_OBrien @mvankerkhove "My concern right now is that this virus has huge kinetic energy”, says @DrMikeRyan, driven by human behaviour, variants and other factors.
“We're pushing three different accelerators at the same time, and we expect the virus to slow down.”
“We're pushing three different accelerators at the same time, and we expect the virus to slow down.”
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan @Kate_L_OBrien @mvankerkhove “Some countries are at such a rate of infection that their health systems are back under severe pressure”, says @DrMikeRyan. “We've seen the tragedy in India. We need to avoid that same tragedy occurring in other countries and some other countries are heading in that direction."
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan @Kate_L_OBrien @mvankerkhove "I have exactly the same hope that we will eventually get to vaccination coverage rates that will protect everybody, but we're not there yet”, says @DrMikeRyan. "We are simply not there yet."
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan @Kate_L_OBrien @mvankerkhove Variants may be contributing to spike in infections, says @DrMikeRyan, but it is driven by crowding and mixing of people, poor ventilation, no masks. etc.
"I know how hard that is in the context of some countries, but that is the brutal reality."
"I know how hard that is in the context of some countries, but that is the brutal reality."
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan @Kate_L_OBrien @mvankerkhove “Some countries really need to take a look in the mirror in terms of what they are doing to drive down infection rates”, says @DrMikeRyan. "Otherwise health care systems in many countries will face real issues in the coming weeks and months."
@DrTedros @WHO @nytimes @MazzucatoM @DrMikeRyan @Kate_L_OBrien @mvankerkhove "I think at this point, many of us may have expected we'd be coming out of this pandemic and we'd be thinking about the future.
We're not.
We're still in the heart of this thing”, says @DrMikeRyan.
We're not.
We're still in the heart of this thing”, says @DrMikeRyan.
• • •
Missing some Tweet in this thread? You can try to
force a refresh