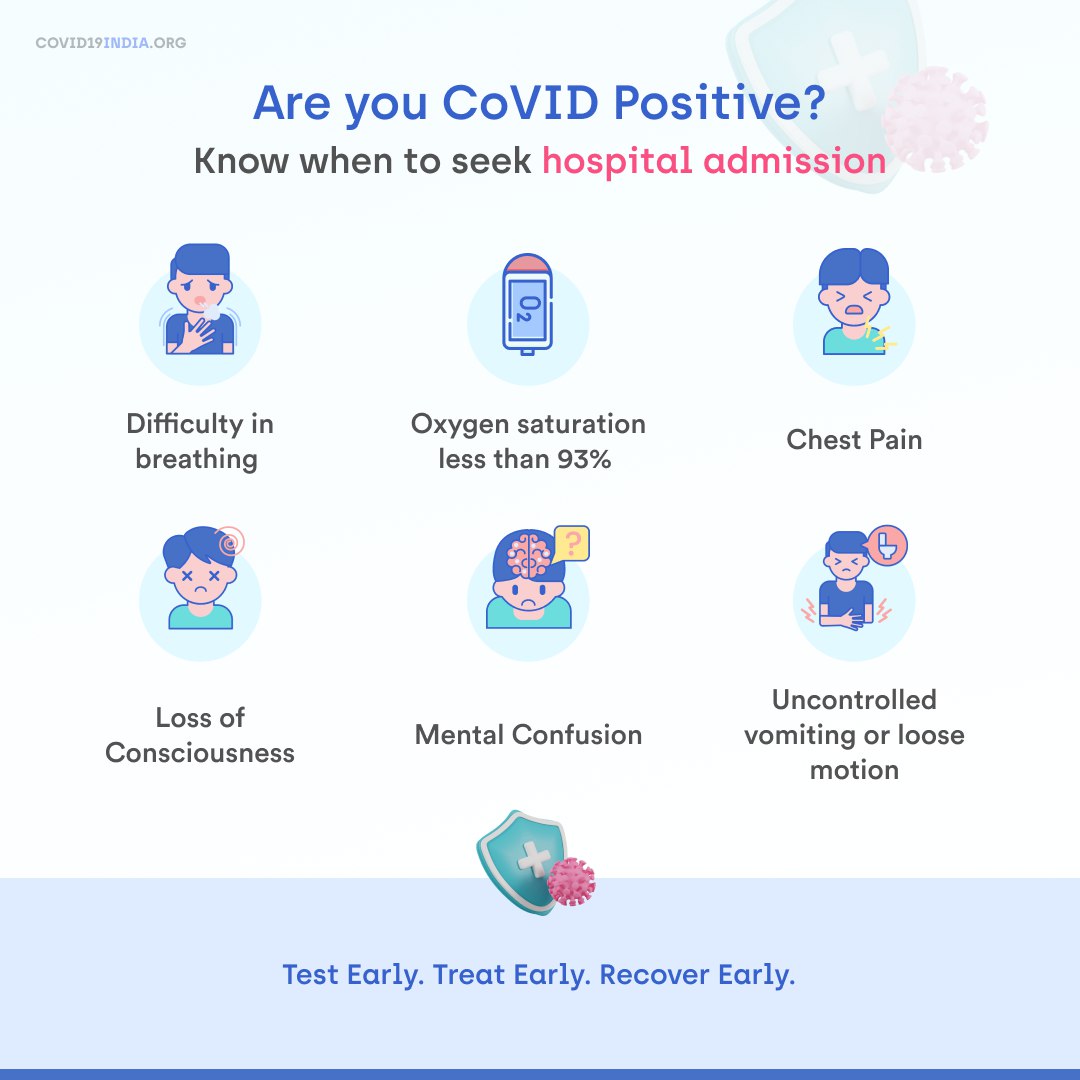
As Covid spread to rural India, can you help build awareness?
Here are #Covid19 awareness posters in 6 vernacular languages:
✅ Hindi, Bengali, Kannada, Malayalam, Odia and Tamil.
Awareness can save lives.
Share the word.
Help save lives!
#COVID #COVID19India



Here are #Covid19 awareness posters in 6 vernacular languages:
✅ Hindi, Bengali, Kannada, Malayalam, Odia and Tamil.
Awareness can save lives.
Share the word.
Help save lives!
#COVID #COVID19India




#malayalam
Stay aware about Corona.
Awareness can save lives. Help spread the word. Help save lives.



Stay aware about Corona.
Awareness can save lives. Help spread the word. Help save lives.




• • •
Missing some Tweet in this thread? You can try to
force a refresh