How big of a wave of #COVID19 do we expect in the US from the Delta variant? Here I describe a simple approach to this question and attempt a rough back-of-the-envelop estimate. 1/16
First off, epidemic size is determined by two primary factors:
1. Efficiency of onward transmission from an index case, commonly quantified as R0
2. Size of susceptible pool
2/16
1. Efficiency of onward transmission from an index case, commonly quantified as R0
2. Size of susceptible pool
2/16
Given a specified R0, we can calculate final epidemic size in a simple SIR model with the following equation where Z is final epidemic size. For initial R0 of 1.1, an epidemic is expected to infect 18% of the susceptible population. 3/16 

Before we get to Delta, we can see how this logic works with the fall/winter wave in the US. Here, state-level estimates of Rt were ~1.1 in Sep and Oct 2020 and declined to ~0.9 in Mar 2021 as the epidemic ran its course and left a wake of population immunity. 4/16
From Sep through Mar, the US reported 24M cases of COVID-19. If we assume 2.7 infections per reported case (via covid19-projections.com) we arrive at 65M infections in a population of 328M for ~20% of the US infected in the fall/winter wave. 5/16
Notably, the (super rough) math seems to work here. Initial Rt of ~1.1 translated to ~20% of the population infected, which is close to the 18% mathematical expectation. 6/16
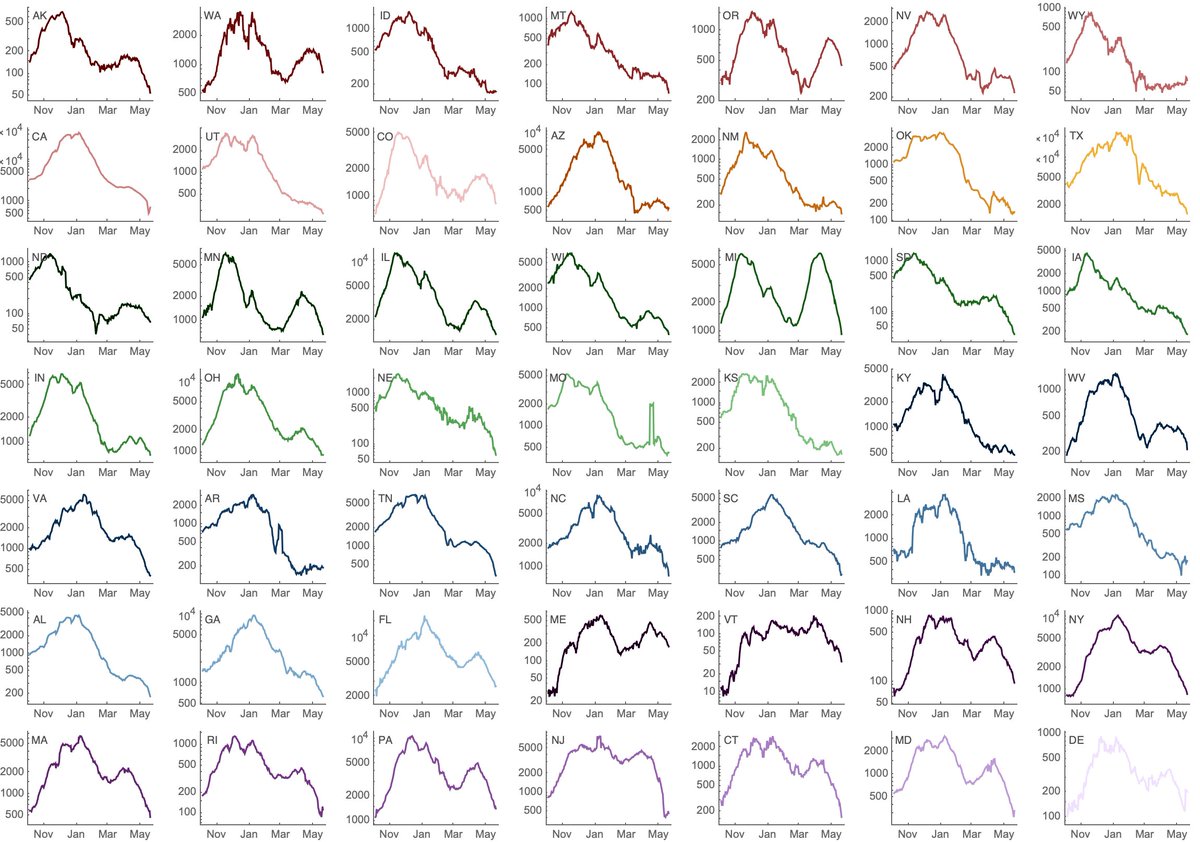
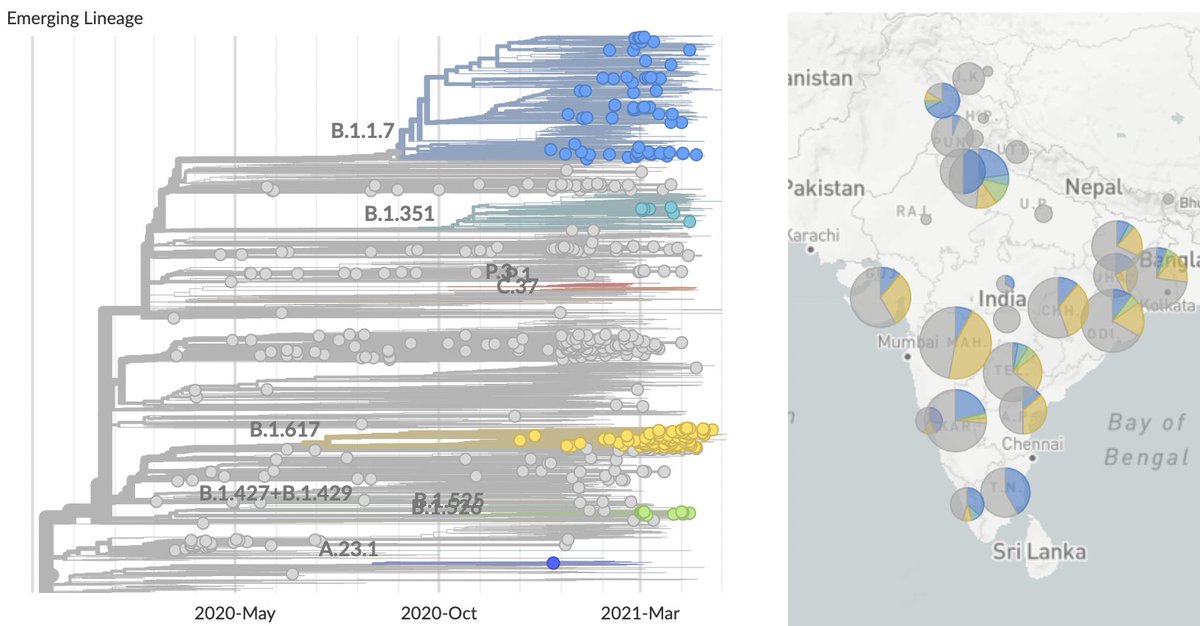
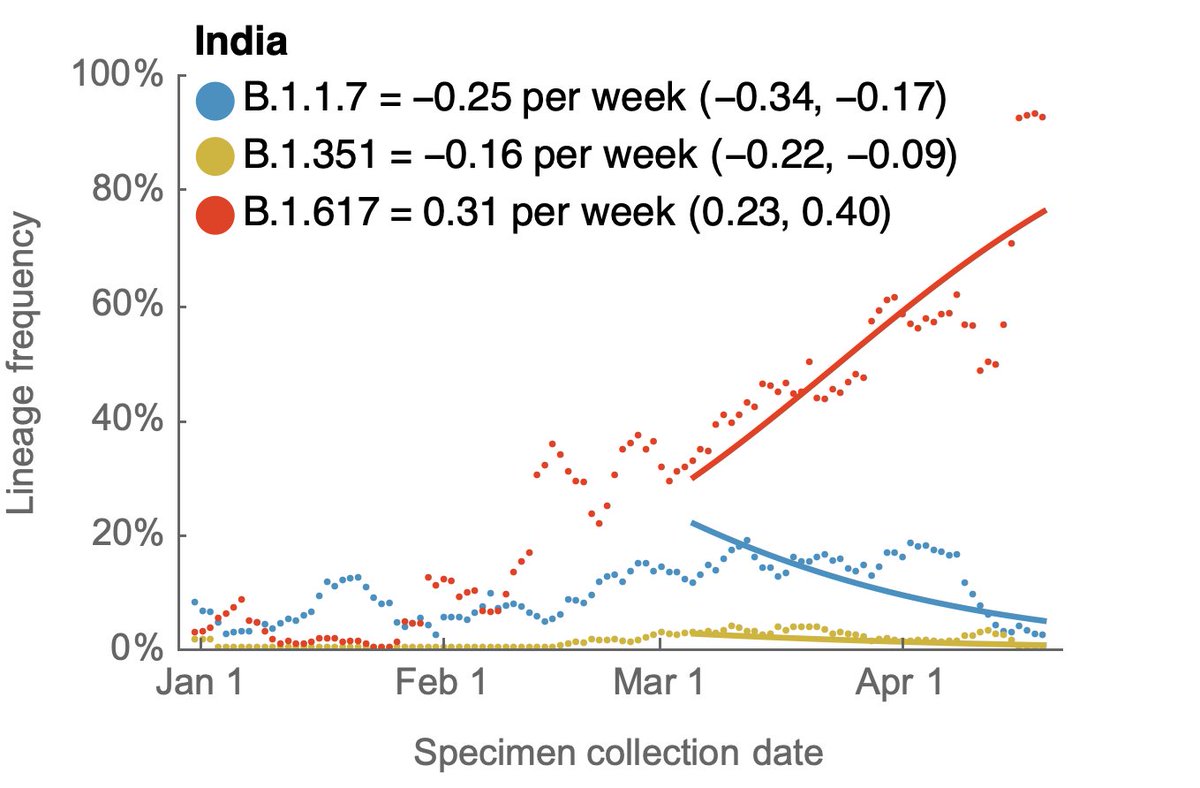
We've recently seen rapid growth of Delta, where it's displacing other circulating viruses in many states. Here, predominance of Delta in Arkansas, Colorado, Missouri and Utah occurred in only ~4 weeks. 7/16 

Variant-specific estimates of Rt from @marlinfiggins using @CDCgov case counts and @GISAID sequence data suggests Rt of Delta variant of 1.18 averaged across 20 states analyzed. 8/16 

Inputting R0 of 1.18 in the final epidemic size equation yields 29% of the susceptible population infected in the Delta wave. 9/16
CDC lists 46% of the US population as fully vaccinated. Following covid19-projections.com, we take ~30% of the US population as having been infected. Assuming that vaccination and infection are independent yields 46% + 54% x 30% = 62% of the population with immunity. 10/16
This would imply that Delta has a susceptible pool of 38% of the US population and given final size of 29% would suggest 11% of the total US population infected with Delta or 36M people. 11/16
A couple obvious places where this calculation is wrong. First, vaccination and infection are not independent and instead probably anti-correlated. This would imply a larger fraction of the population immune than the 62% used above. 12/16
Second, vaccination is not perfect and vaccine efficacy against symptomatic disease drops from ~89% against Alpha to ~79% against Delta per @PHE_uk report (assets.publishing.service.gov.uk/government/upl…). Breakthrough infections imply a smaller fraction of the population immune than the 62%. 13/16 

I'd hope for these two effects to largely come out in the wash. 14/16
Another 11% of the population infected is of course substantial, though we expect lower rates of mortality on a per case basis due to vaccination coverage differing substantially across age groups (cdc.gov/mmwr/volumes/7…). 15/16 

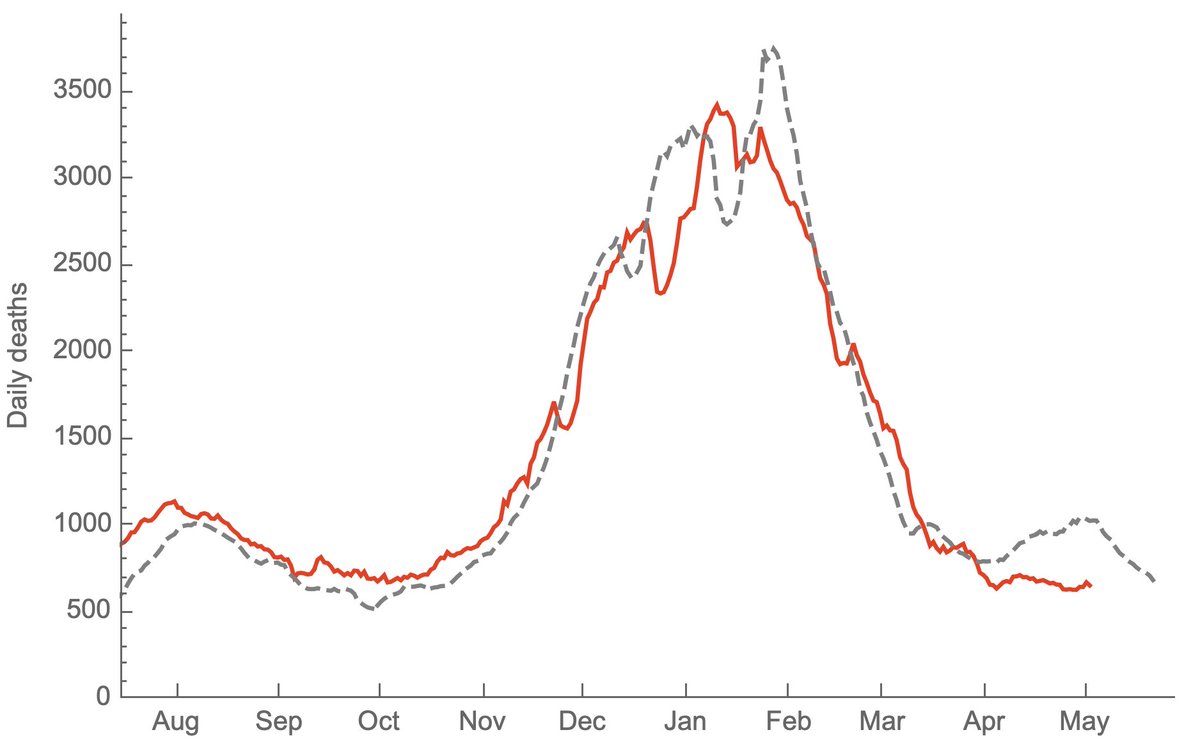
Although I don't have a huge amount of faith in this specific 11% estimate, at the moment, just based on what's happening in the UK, I would expect there to be a US wave driven by Delta (figure showing Rt in UK from epiforecasts.io/covid/posts/na…). 16/16 

Follow up #1: Prompted by question on Twitter (
https://twitter.com/Nutmeg031992/status/1410409021492936704) I just did a simple correlation of total population fully vaccinated against Delta-specific Rt across 20 states with good data. We get a decently strong negative correlation with R^2 of 0.32.

Follow up #2: This supports the expectation that we'll see Delta circulation influenced by degree of regional vaccine coverage.
https://twitter.com/trvrb/status/1407466078834360320
• • •
Missing some Tweet in this thread? You can try to
force a refresh