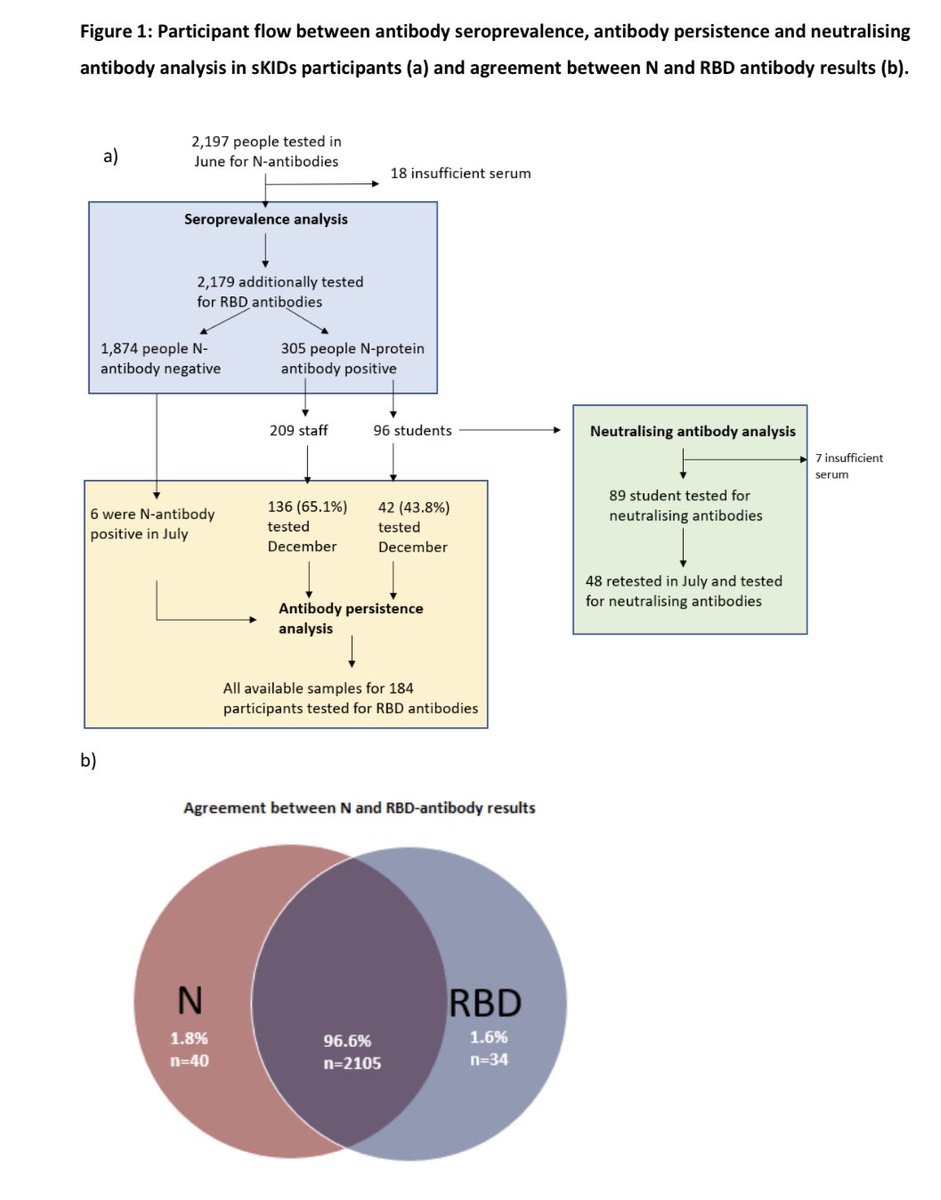
1/ It was the JCVI that used basic immunological principles of vaccination to recommend a 12-week interval between #COVID19 vaccines to save more lives during the Alpha wave in the UK
Our paper (preprint) here shows why that was the right decision…🧵
👉medrxiv.org/content/10.110…
Our paper (preprint) here shows why that was the right decision…🧵
👉medrxiv.org/content/10.110…
2/ We tested #SARSCoV2 antibodies in adults aged 50-89 years and found that, for both Pfizer & AZ vaccines, 95% had seroconverted (developed antibodies) by 35-55 days after the first #COVID19 dose, and 100% by 7+ days after the second dose
👉 medrxiv.org/content/10.110…
👉 medrxiv.org/content/10.110…
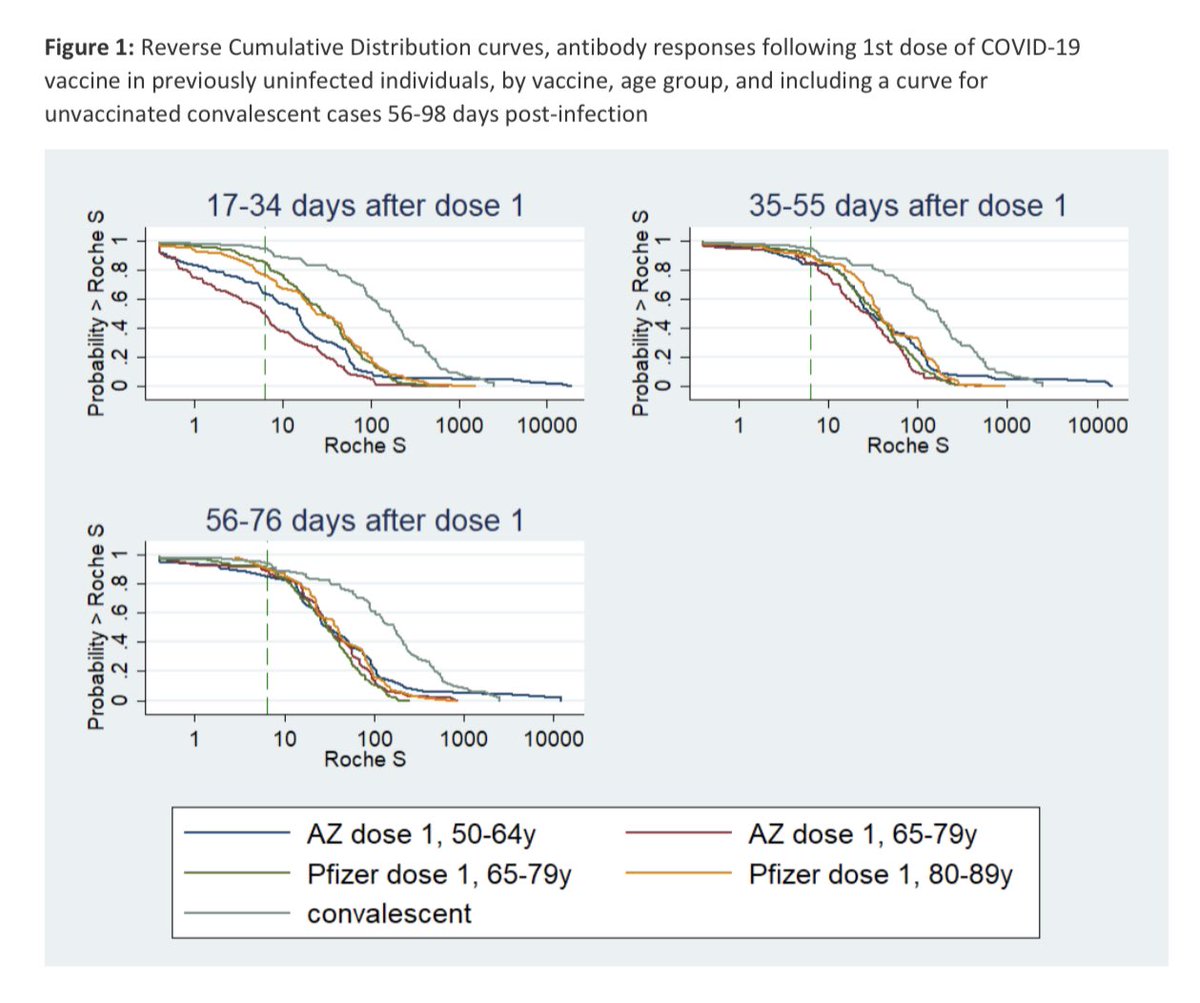
3/ BUT, when the vaccines were given 9-11 weeks apart, antibody levels at 2-5 weeks after 2nd dose were 6x ⬆️ for Pfizer (6703; 95%CI, 5887-7633) than AZ (1093; 806-1483), which in turn were higher than Pfizer given 3-4 weeks apart (694; 540 - 893) 👉medrxiv.org/content/10.110… 

4/ Across England, for both #COVID19 vaccines, effectiveness (VE) was ⬆️ in all age-gps from 14 days after dose 2 vs dose 1, but varied with dose interval, with higher 2-dose VE when Pfizer doses were given >6 weeks apart compared to a 3-week schedule, including ≥80 year-olds 
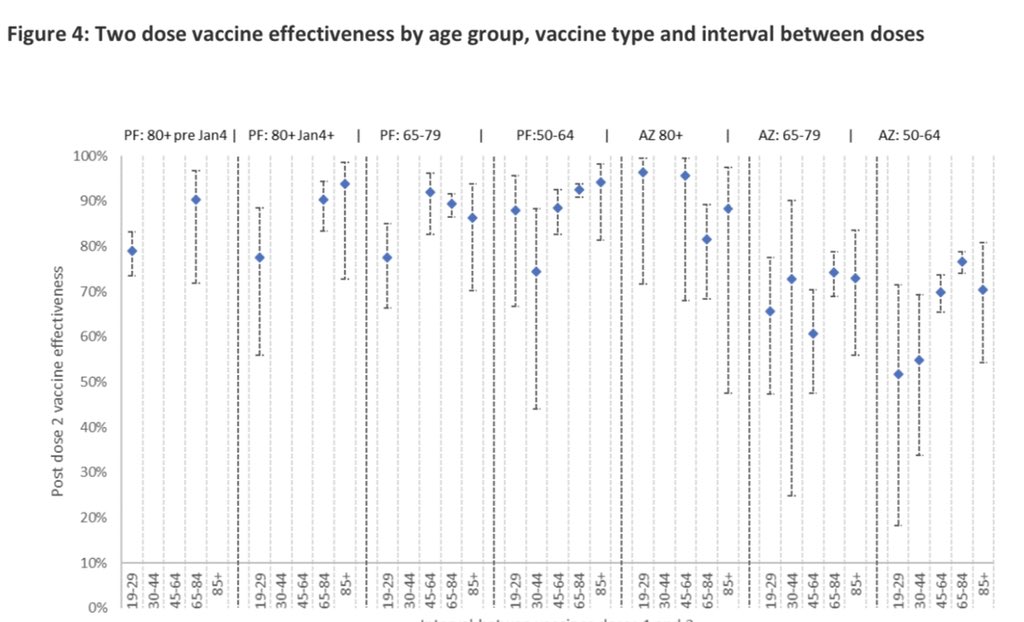
5/ When assessing vaccine effectiveness (VE) by interval between the two #COVID19 vaccine doses, the “sweet spot” does seem to be around the 8 week interval for the two vaccines, which supports the recent national recommendations to reduce the dose interval from 12 to 8 weeks 

6/ In summary,
- The violin plot👇 shows nicely how extending interval between #COVID19 vaccine doses improves immune response after 2nd dose
- Remember that extending dose interval to 12 weeks meant many more older adults could be vaccinated quicker with limited vaccine supply
- The violin plot👇 shows nicely how extending interval between #COVID19 vaccine doses improves immune response after 2nd dose
- Remember that extending dose interval to 12 weeks meant many more older adults could be vaccinated quicker with limited vaccine supply

• • •
Missing some Tweet in this thread? You can try to
force a refresh