1/
Should we use #molnupiravir (MOV) to treat #SARSCoV2 #COVID19?
With new @NIH treatment guidelines out, it's time to review the evidence behind them.
Here's #HowIReadThisPaper on the MOVe-OUT trial by Bernal et al in @NEJM
(Thread)
nejm.org/doi/full/10.10…
Should we use #molnupiravir (MOV) to treat #SARSCoV2 #COVID19?
With new @NIH treatment guidelines out, it's time to review the evidence behind them.
Here's #HowIReadThisPaper on the MOVe-OUT trial by Bernal et al in @NEJM
(Thread)
nejm.org/doi/full/10.10…
2/
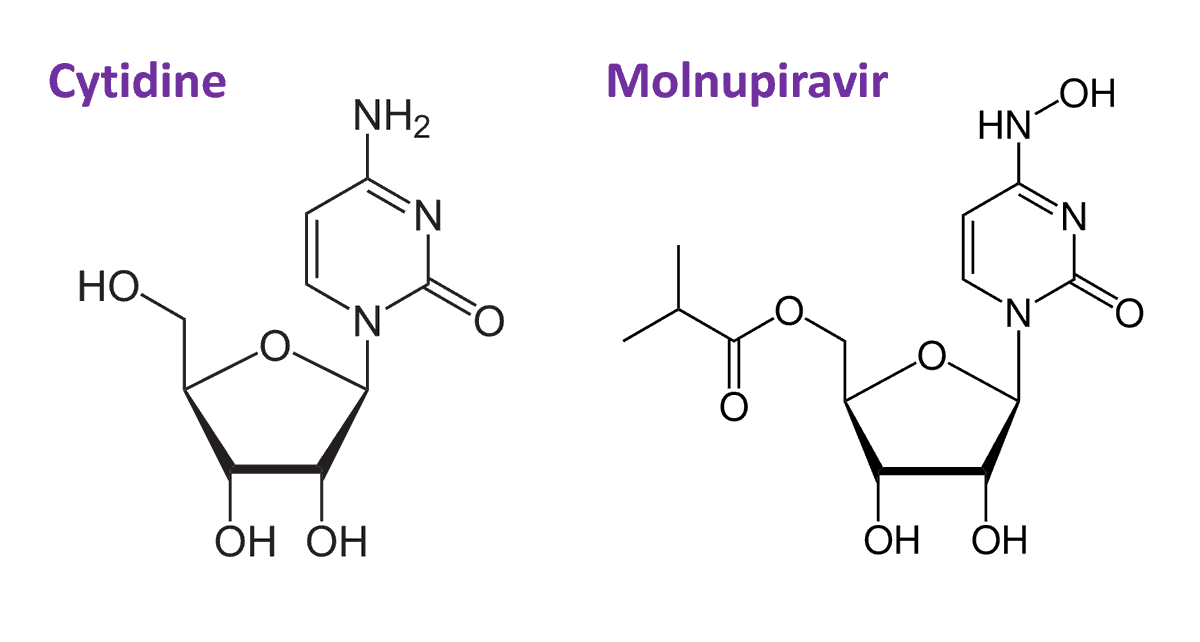
How does this drug work?
MOV is a RNA nucleotide prodrug of n-hydroxycytidine, a cytidine analogue that is a potent mutagen for viruses.
It works by tricking viral RNA polymerase into making errors during replication.
nature.com/articles/s4159…
How does this drug work?
MOV is a RNA nucleotide prodrug of n-hydroxycytidine, a cytidine analogue that is a potent mutagen for viruses.
It works by tricking viral RNA polymerase into making errors during replication.
nature.com/articles/s4159…
3/
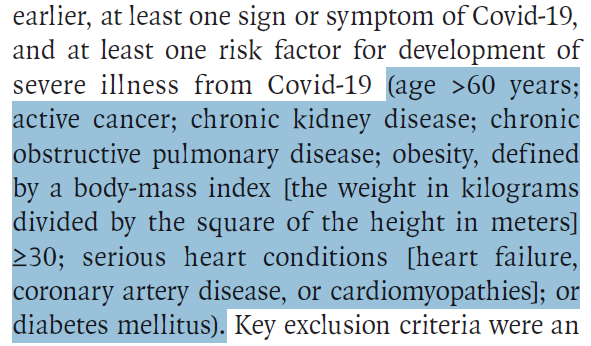
In which patients was this drug studied?
1,433 unvaccinated outpatients
>18 years old
with symptomatic #COVID19
for <5 days
and at least one risk factor for severe disease:
In which patients was this drug studied?
1,433 unvaccinated outpatients
>18 years old
with symptomatic #COVID19
for <5 days
and at least one risk factor for severe disease:
4/
Patients were enrolled all over the world.
Median age in the 40s
Most common comorbidities: obesity, age >60, DM2
20% had prior infection (antibodies)
Half had Sx onset < 3 days
Most common variant: delta
Patients were enrolled all over the world.
Median age in the 40s
Most common comorbidities: obesity, age >60, DM2
20% had prior infection (antibodies)
Half had Sx onset < 3 days
Most common variant: delta

5/
Patients were randomized to placebo or MOV x5 days.
Randomization was stratified by Sx duration <3 days
Primary outcome: absolute risk reduction (ARR) in all-cause hospitalization or death by day 29
Patients were randomized to placebo or MOV x5 days.
Randomization was stratified by Sx duration <3 days
Primary outcome: absolute risk reduction (ARR) in all-cause hospitalization or death by day 29
6/
What were the main findings?
Primary outcome:
Interim ARR: ↓ 6% in hospitalizations & death
Final ARR: ↓ 3% in hospitalizations & death
Expressed as time-to-event:
HR 0.69 [95% CI 0.48-1.01]
Note that this confidence interval includes 1 (i.e., no effect)
What were the main findings?
Primary outcome:
Interim ARR: ↓ 6% in hospitalizations & death
Final ARR: ↓ 3% in hospitalizations & death
Expressed as time-to-event:
HR 0.69 [95% CI 0.48-1.01]
Note that this confidence interval includes 1 (i.e., no effect)

7/
This was technically a positive study, but no one seems excited about MOV.
@NIH ranked it in last place in order of preference:
covid19treatmentguidelines.nih.gov/therapies/stat…
Why?
Let’s examine sources of chance and bias that could help us understand the results.
This was technically a positive study, but no one seems excited about MOV.
@NIH ranked it in last place in order of preference:
covid19treatmentguidelines.nih.gov/therapies/stat…
Why?
Let’s examine sources of chance and bias that could help us understand the results.
8/
First, gender balance in the treatment groups was unequal.
By chance, more women were in the MOV group, and more men were in the placebo group.
We know men are at ↑ risk for severe disease.
This could bias towards an effect of MOV.
(that is, gender could be a confounder)

First, gender balance in the treatment groups was unequal.
By chance, more women were in the MOV group, and more men were in the placebo group.
We know men are at ↑ risk for severe disease.
This could bias towards an effect of MOV.
(that is, gender could be a confounder)


9/
In fact, controlling for gender completely attenuated the association, and the primary outcome no longer reached statistical significance:
Adjusted ARR: ↓ 2.8% (95% CI, −5.7 to 0.1)
This means the benefit of MOV could be completely explained by the gender imbalance.
In fact, controlling for gender completely attenuated the association, and the primary outcome no longer reached statistical significance:
Adjusted ARR: ↓ 2.8% (95% CI, −5.7 to 0.1)
This means the benefit of MOV could be completely explained by the gender imbalance.
10/
Second, the subgroup analyses support that the effect size is small.
Two subgroups are particularly informative:
Sx duration < 3 days
Antibody status (prior infection)
Second, the subgroup analyses support that the effect size is small.
Two subgroups are particularly informative:
Sx duration < 3 days
Antibody status (prior infection)

11/
There was no benefit of MOV in the subgroup of patients with shorter symptoms.
Being an antiviral, if there was a real benefit to MOV, this is where you’d expect to see it.
Early benefit is the case with remdesivir and antivirals for other diseases like influenza.
There was no benefit of MOV in the subgroup of patients with shorter symptoms.
Being an antiviral, if there was a real benefit to MOV, this is where you’d expect to see it.
Early benefit is the case with remdesivir and antivirals for other diseases like influenza.
12/
Also, benefit was concentrated in patients without prior infection.
This is not surprising, but it is informative:
If the immunity of prior infection is enough to attenuate the effect of MOV, this suggests the benefit is substantially less than that from vaccination.
Also, benefit was concentrated in patients without prior infection.
This is not surprising, but it is informative:
If the immunity of prior infection is enough to attenuate the effect of MOV, this suggests the benefit is substantially less than that from vaccination.
13/
Why did the effect size drop by 50% from the interim to the final analysis?
This is not really clear to me. I would love to hear any ideas.
One possibility is that the highest risk patients were already vaccinated and thus ineligible for the trial.
Why did the effect size drop by 50% from the interim to the final analysis?
This is not really clear to me. I would love to hear any ideas.
One possibility is that the highest risk patients were already vaccinated and thus ineligible for the trial.
14/
Further, long-term risks of mutagenesis are not addressed in this study.
Given its mechanism, there is also some concern about MOV causing teratogenicity in humans.
In fact, the FDA EUA recommends contraception for 3 months after the last dose:
fda.gov/news-events/pr…
Further, long-term risks of mutagenesis are not addressed in this study.
Given its mechanism, there is also some concern about MOV causing teratogenicity in humans.
In fact, the FDA EUA recommends contraception for 3 months after the last dose:
fda.gov/news-events/pr…

15/
In light of these problems, MOV is recommended for use only if no other options are available.
Note that other recommended drugs (e.g. remdesivir, sotrovimab) and as-yet not-recommended drugs (e.g. fluvoxamine) have performed as well or better, with fewer safety concerns.
In light of these problems, MOV is recommended for use only if no other options are available.
Note that other recommended drugs (e.g. remdesivir, sotrovimab) and as-yet not-recommended drugs (e.g. fluvoxamine) have performed as well or better, with fewer safety concerns.
16/
One important silver lining:
Because MOV targets the viral RNA polymerase, mutations in the spike protein between different variants are unlikely to affect its efficacy.
This could make MOV a valuable tool for future variants with a high degree of immune escape.
One important silver lining:
Because MOV targets the viral RNA polymerase, mutations in the spike protein between different variants are unlikely to affect its efficacy.
This could make MOV a valuable tool for future variants with a high degree of immune escape.
17/
Bottom line:
MOV modestly reduced hospitalizations and death at day 29 in high-risk outpatients with #COVID19.
However, this benefit could be entirely due to confounding by gender.
Subgroup analyses suggest any real benefit is likely to be very small.
(End)
Bottom line:
MOV modestly reduced hospitalizations and death at day 29 in high-risk outpatients with #COVID19.
However, this benefit could be entirely due to confounding by gender.
Subgroup analyses suggest any real benefit is likely to be very small.
(End)
18/
My deepest thanks to @branchwestyn and Dr. Amanda Westlake for their thoughtful peer review.
If you haven't already, please read the paper and contribute to the discussion below!
My deepest thanks to @branchwestyn and Dr. Amanda Westlake for their thoughtful peer review.
If you haven't already, please read the paper and contribute to the discussion below!
• • •
Missing some Tweet in this thread? You can try to
force a refresh