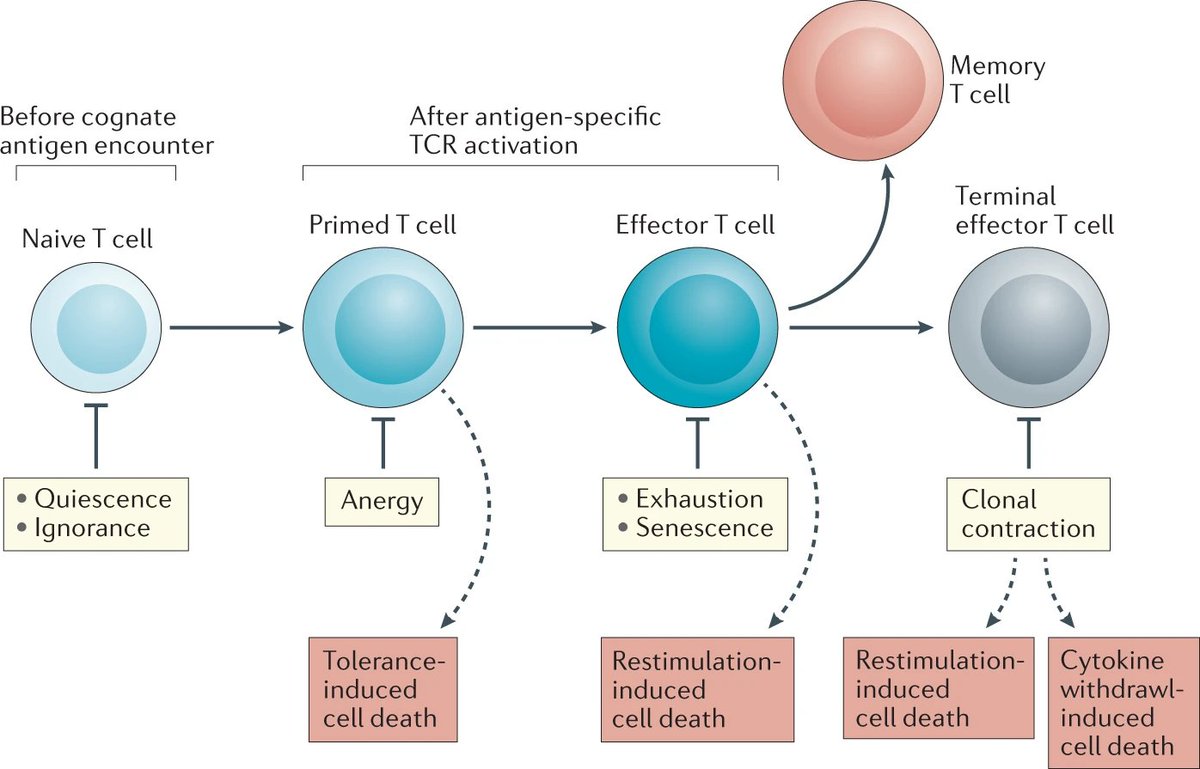
(1) A lot of Immunologists are (justifiably) obsessed with T cell exhaustion.
Very few discuss memory inflation 📈 despite its relevance to #vaccines and #aging
#Immunology #TCELL
Diagram and background info mainly from bit.ly/OHara2012
Very few discuss memory inflation 📈 despite its relevance to #vaccines and #aging
#Immunology #TCELL
Diagram and background info mainly from bit.ly/OHara2012

(2) Inflation: induction of memory [CD8+] T cells that increase in frequency over time to >10% of the entire T cell pool in blood and higher abundance in peripheral tissues (e.g. liver and lung)
(3) First reported (to my knowledge) in a mouse model of CMV (MCMV)
Recall (by contrast) how chronic LCMV (clone 13) induces T cell exhaustion
bit.ly/Holtappels2000
bit.ly/Karrer2003
Recall (by contrast) how chronic LCMV (clone 13) induces T cell exhaustion
bit.ly/Holtappels2000
bit.ly/Karrer2003
(4) But also relevant to the human T cell response and repertoire composition. For example, HCMV-specific memory T cells represent ~10% of the circulating memory (CD4 and CD8) compartment, which can increase further with age
bit.ly/Komatsu2003
bit.ly/Sylwester2005

bit.ly/Komatsu2003
bit.ly/Sylwester2005

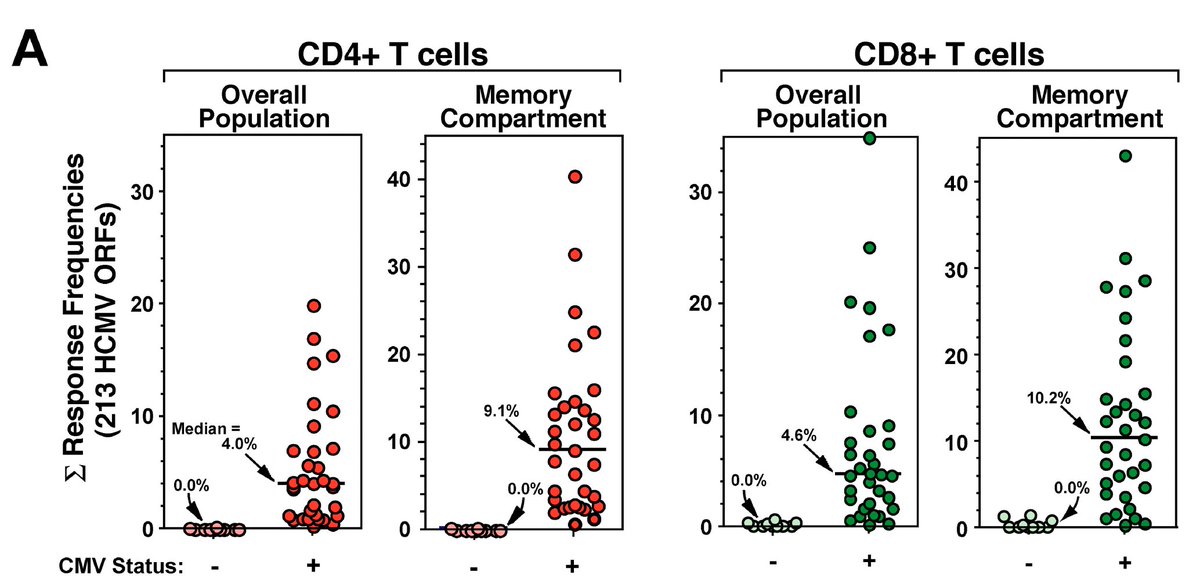
(5) Two facts:
1-It appears that memory inflation is induced by persistent (latent) viruses
2-Of note, these memory T cells do NOT display features of exhaustion
jimmunol.org/content/187/4/…
jci.org/articles/view/…
bit.ly/Snyder2008
1-It appears that memory inflation is induced by persistent (latent) viruses
2-Of note, these memory T cells do NOT display features of exhaustion
jimmunol.org/content/187/4/…
jci.org/articles/view/…
bit.ly/Snyder2008
(6) My speculation is that persistent "latent" viruses support memory inflation whereas persistent "active" viruses promote exhaustion 

(7) Examples of viruses (aside from HCMV) that induce memory inflation
Systemic HSV
bit.ly/Lang2009
bit.ly/Lang2011
Murine polyomavirus (PyV)
bit.ly/Swanson2009
bit.ly/Swanson2008

Systemic HSV
bit.ly/Lang2009
bit.ly/Lang2011
Murine polyomavirus (PyV)
bit.ly/Swanson2009
bit.ly/Swanson2008


(8)
Acute parvovirus B19 infection
bit.ly/Isa2005
bit.ly/Norbeck2005
Parvovirus PARV4
bit.ly/Simmons2013
Acute parvovirus B19 infection
bit.ly/Isa2005
bit.ly/Norbeck2005
Parvovirus PARV4
bit.ly/Simmons2013
(9) Okay, how is this relevant to vaccines?
Here is where it gets interesting!
~ a decade ago, @KlenermanLab and @ImmunobiologySG developed a model of inducing memory T cell inflation via immunizing with a replication-deficient adenovirus-based vector
bit.ly/Bolinger2013
Here is where it gets interesting!
~ a decade ago, @KlenermanLab and @ImmunobiologySG developed a model of inducing memory T cell inflation via immunizing with a replication-deficient adenovirus-based vector
bit.ly/Bolinger2013

(10)Aside from promising a better future for vaccines, , the second interesting insight was showing the induction of "conventional" but not "inflating" ag-specific CD8 T cell memory was dependent upon the expression of immunoproteasome components (involved in antigen processing) 

(11)that are uniquely expressed by professional antigen-presenting cells. This finding provoked the hypothesis that perhaps the critical APC involved in memory inflation MAY not be professional APCs but STROMAL cells 😯🙄 (!)
A review on stromal cells bit.ly/Onder2021
A review on stromal cells bit.ly/Onder2021
(12)Last year, the same group utilized a genetic approach to resolve this question.
They employed a similar Ad5-based vector but β-gal expression was now dependent on Cre recombinase activity
go.nature.com/3Meca4x

They employed a similar Ad5-based vector but β-gal expression was now dependent on Cre recombinase activity
go.nature.com/3Meca4x


(13) By expressing Cre in different populations, the authors proved that targeting β-gal expression to myeloid cells (LySM-Cre and Cd11c-Cre) did NOT drive inflating or conventional CD8+ T cell responses!!! 

(14) Targeting β-gal expression only to Ccl19+ cells resulted in strong inflationary (and conventional) responses.
Ccl19 is expressed by fibroblastic stromal cells (FSCs) in tissues known to support the survival of lymphocytes and provide the architectural context for immunity
Ccl19 is expressed by fibroblastic stromal cells (FSCs) in tissues known to support the survival of lymphocytes and provide the architectural context for immunity

(15) Using a strategy to deplete the relevant FSCs using a DTR approach, intranasal treatment after Ad infection which depletes Ccl19-expressing FSCs selectively in the lungs, compromised the inflating response to the same degree as that observed with the systemic depletion. 

(16) Of course, it is never simple.
There was a secondary role for professional APCs in memory inflation since β-gal expression in FSCs was insufficient to provoke memory inflation in mice lacking dendritic cells capable of antigen cross-presentation
As well as FSC IL-33
There was a secondary role for professional APCs in memory inflation since β-gal expression in FSCs was insufficient to provoke memory inflation in mice lacking dendritic cells capable of antigen cross-presentation
As well as FSC IL-33
(17) The reason I am tweeting all of this is that we mostly tend to focus on studying instances of immune system underachievement (e.g. exhaustion) and tend to ignore cases where the immune system overachieves
Mechanistically learning from both can help tailor better vaccines
Mechanistically learning from both can help tailor better vaccines
(18) For a more eloquent and comprehensive perspective, check out Block & Jameson nature.com/articles/s4159… 

• • •
Missing some Tweet in this thread? You can try to
force a refresh