(1) The plasma cell (PC) field has been booming and blooming in the past two years
Some dogmas and notions were quietly taken apart
An #immunology 🧵
Some dogmas and notions were quietly taken apart
An #immunology 🧵
(2) First, a pervasive dogma was that germinal centers are the sole origin of long-lived PCs
There is accumulating evidence that LLPCs can originate from T-cell-independent (extrafollicular) responses
bit.ly/Fidler1975
bit.ly/Bortnik2012
bit.ly/Bortnik2013
There is accumulating evidence that LLPCs can originate from T-cell-independent (extrafollicular) responses
bit.ly/Fidler1975
bit.ly/Bortnik2012
bit.ly/Bortnik2013

(3) Even within a GC-derived response, if you genetically label PCs prior to GC formation, you still get Long-lived plasma cells during this nascent extrafollicular response
bit.ly/Koike2023
Koike et al. here elegantly making use of two "timestamping" genetic tools
bit.ly/Koike2023
Koike et al. here elegantly making use of two "timestamping" genetic tools

(4) Hai Qi and co. saw a similar IgM long-lived PC germline (no somatic hypermutations) repertoire enriched in public clones supporting the studies above
bit.ly/Liu2022
bit.ly/Liu2022

(6) One could have presumed this answer in the light of evolution since lampreys do not have germinal centers (personal communication) and indeed retain the features of long-lived antibody immunity (and live for ≤ 8 years)
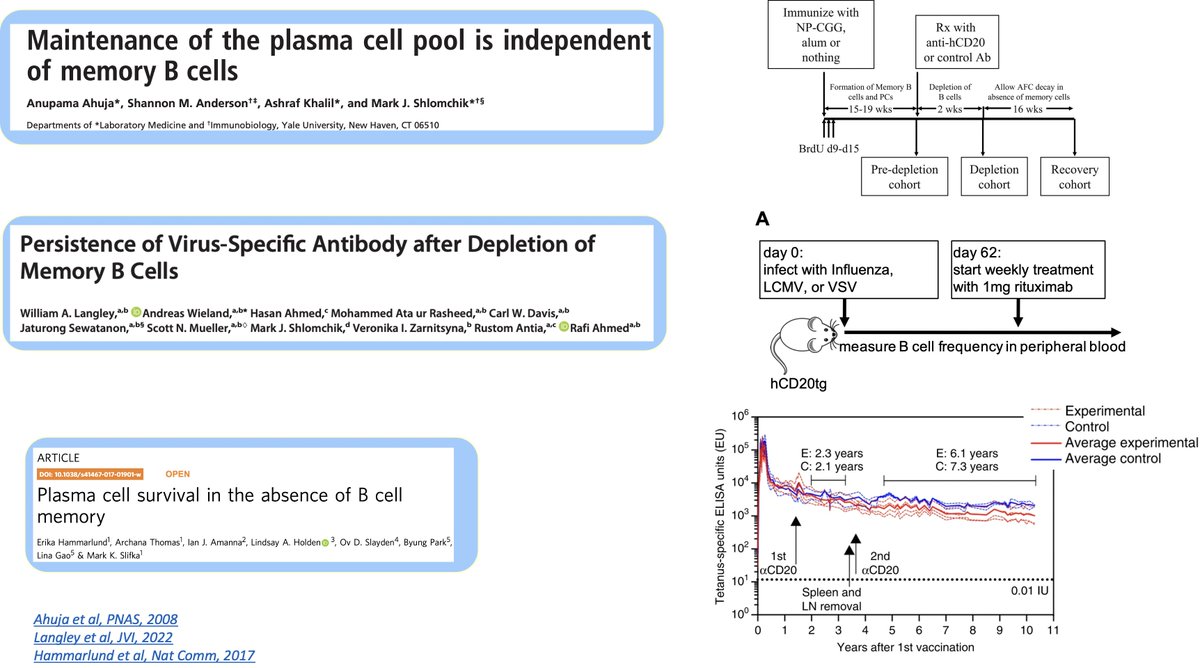
(7) The second pervasive (rather intuitive) notion is that memory B cells replenish the long-lived PC pool based on a high-profile study using in vitro human memory B cells
(8) However, several studies in mice and macaques definitely dispelled this suggestion
via anti-CD20 depleting memory B cells and even removing lymph nodes and spleen.
bit.ly/Ahuja2008
bit.ly/Langley2022
bit.ly/Hammarlund2017
via anti-CD20 depleting memory B cells and even removing lymph nodes and spleen.
bit.ly/Ahuja2008
bit.ly/Langley2022
bit.ly/Hammarlund2017
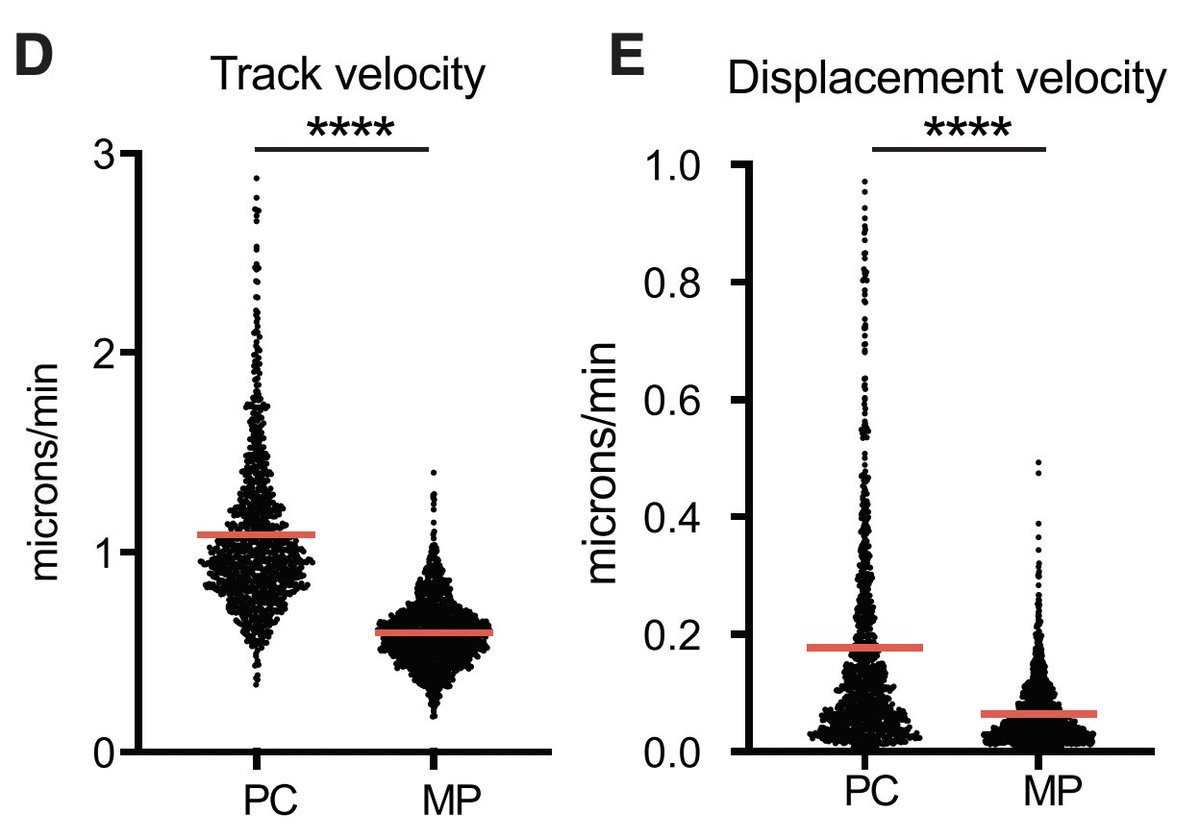
(9) The third dogma is that plasma cells once they find their "effector" sites (i.e. bone marrow or intestines...etc) become immotile and just sit around 

(10) Fooksman and colleagues show in a paradigm-shifting study that BM PCs recirculate to other bones and the spleen even in the absence of immunization
At the span of hours (heroic intravital imaging)
bit.ly/Benet2021

At the span of hours (heroic intravital imaging)
bit.ly/Benet2021

(11) Some of these less motile plasma cells cluster around areas of high APRIL (survival cytokine) density
These are enriched in LLPCs
biorxiv.org/content/10.110…
These are enriched in LLPCs
biorxiv.org/content/10.110…

(12) Another prevailing notion was that mature plasma cells no longer have competent B cell receptors
Which is intuitive and stems from the CORRECT findings that
A-LLPC (IgG) PCs don't need antigen to survive
bit.ly/Manz1998
Which is intuitive and stems from the CORRECT findings that
A-LLPC (IgG) PCs don't need antigen to survive
bit.ly/Manz1998

(13)
B-LLPCs downregulate their surface BCR in favor of the secreted antibody (Also TRUE)
However, it turns out that mature IgM PCs express both surface BCR and the coreceptor CD79
bit.ly/Blanc2016
B-LLPCs downregulate their surface BCR in favor of the secreted antibody (Also TRUE)
However, it turns out that mature IgM PCs express both surface BCR and the coreceptor CD79
bit.ly/Blanc2016

(14) Not just IgM, but also IgA and IgE PCs...Actually, Allen and co. recently showed that BCR engagement by IgE PCs triggers their death 🪦
bit.ly/Wade-Vallance2…
bit.ly/Wade-Vallance2…

(15) This finding may help explain why mice and humans have so few IgE plasma cells
It may be that they are continuously eliminated by what could help your conventional B cell survive.
It may be that they are continuously eliminated by what could help your conventional B cell survive.
(16) So the point is, some PCs still express surface BCRs that can competently signal...What does it mean?
Not sure yet.
Not sure yet.
(17) There remains 3 more intriguing phenomena that go against prevailing dogma, but I guess we should wait for these to be published by the various groups interested in plasma cells before musing on this platform.
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter